Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation
- PMID: 20152117
- PMCID: PMC3110675
- DOI: 10.1016/j.neuron.2009.12.005
Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation
Abstract
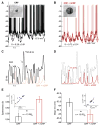
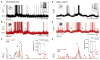
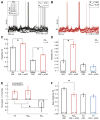
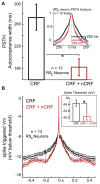
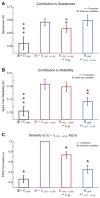
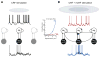
During natural vision, the entire visual field is stimulated by images rich in spatiotemporal structure. Although many visual system studies restrict stimuli to the classical receptive field (CRF), it is known that costimulation of the CRF and the surrounding nonclassical receptive field (nCRF) increases neuronal response sparseness. The cellular and network mechanisms underlying increased response sparseness remain largely unexplored. Here we show that combined CRF + nCRF stimulation increases the sparseness, reliability, and precision of spiking and membrane potential responses in classical regular spiking (RS(C)) pyramidal neurons of cat primary visual cortex. Conversely, fast-spiking interneurons exhibit increased activity and decreased selectivity during CRF + nCRF stimulation. The increased sparseness and reliability of RS(C) neuron spiking is associated with increased inhibitory barrages and narrower visually evoked synaptic potentials. Our experimental observations were replicated with a simple computational model, suggesting that network interactions among neuronal subtypes ultimately sharpen recurrent excitation, producing specific and reliable visual responses.
Figures
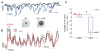

Similar articles
-
Natural stimulation of the nonclassical receptive field increases information transmission efficiency in V1.J Neurosci. 2002 Apr 1;22(7):2904-15. doi: 10.1523/JNEUROSCI.22-07-02904.2002. J Neurosci. 2002. PMID: 11923455 Free PMC article.
-
Clustered organization of neurons with similar extra-receptive field properties in the primary visual cortex.Neuron. 2002 Aug 1;35(3):547-53. doi: 10.1016/s0896-6273(02)00782-1. Neuron. 2002. PMID: 12165475
-
Stimulus-selective spiking is driven by the relative timing of synchronous excitation and disinhibition in cat striate neurons in vivo.Eur J Neurosci. 2008 Oct;28(7):1286-300. doi: 10.1111/j.1460-9568.2008.06434.x. Eur J Neurosci. 2008. PMID: 18973556
-
Mapping the primate lateral geniculate nucleus: a review of experiments and methods.J Physiol Paris. 2014 Feb;108(1):3-10. doi: 10.1016/j.jphysparis.2013.10.001. Epub 2013 Nov 21. J Physiol Paris. 2014. PMID: 24270042 Free PMC article. Review.
-
Circuits that build visual cortical receptive fields.Trends Neurosci. 2006 Jan;29(1):30-9. doi: 10.1016/j.tins.2005.11.001. Epub 2005 Nov 23. Trends Neurosci. 2006. PMID: 16309753 Review.
Cited by
-
γ and the coordination of spiking activity in early visual cortex.Neuron. 2013 Feb 20;77(4):762-74. doi: 10.1016/j.neuron.2012.12.036. Neuron. 2013. PMID: 23439127 Free PMC article.
-
A Physiologically Inspired Model for Solving the Cocktail Party Problem.J Assoc Res Otolaryngol. 2019 Dec;20(6):579-593. doi: 10.1007/s10162-019-00732-4. Epub 2019 Aug 7. J Assoc Res Otolaryngol. 2019. PMID: 31392449 Free PMC article.
-
Interneuronal modulations as a functional switch for cortical computations: mechanisms and implication for disease.Front Cell Neurosci. 2025 Jan 23;18:1479579. doi: 10.3389/fncel.2024.1479579. eCollection 2024. Front Cell Neurosci. 2025. PMID: 39916937 Free PMC article.
-
Modulation of V1 spike response by temporal interval of spatiotemporal stimulus sequence.PLoS One. 2012;7(10):e47543. doi: 10.1371/journal.pone.0047543. Epub 2012 Oct 16. PLoS One. 2012. PMID: 23091631 Free PMC article.
-
Inhibitory interneurons decorrelate excitatory cells to drive sparse code formation in a spiking model of V1.J Neurosci. 2013 Mar 27;33(13):5475-85. doi: 10.1523/JNEUROSCI.4188-12.2013. J Neurosci. 2013. PMID: 23536063 Free PMC article.
References
-
- Anderson JS, Lampl I, Gillespie DC, Ferster D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science. 2000;290:1968–1972. - PubMed
-
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. - PubMed
-
- Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–523. - PubMed
-
- Azouz R, Gray CM, Nowak LG, McCormick DA. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex. 1997;7:534–545. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

