Reverse micelle encapsulation of membrane-anchored proteins for solution NMR studies
- PMID: 20152148
- PMCID: PMC2876244
- DOI: 10.1016/j.str.2009.11.010
Reverse micelle encapsulation of membrane-anchored proteins for solution NMR studies
Abstract
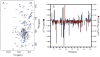
Perhaps 5%-10% of proteins bind to membranes via a covalently attached lipid. Posttranslational attachment of fatty acids such as myristate occurs on a variety of viral and cellular proteins. High-resolution information about the nature of lipidated proteins is remarkably sparse, often because of solubility problems caused by the exposed fatty acids. Reverse micelle encapsulation is used here to study two myristoylated proteins in their lipid-extruded states: myristoylated recoverin, which is a switch in the Ca(2+) signaling pathway in vision, and the myristoylated HIV-1 matrix protein, which is postulated to be targeted to the plasma membrane through its binding to phosphatidylinositol-4,5-bisphosphate. Both proteins have been successfully encapsulated in the lipid-extruded state and high-resolution NMR spectra obtained. Both proteins bind their activating ligands in the reverse micelle. This approach seems broadly applicable to membrane proteins with exposed fatty acid chains that have eluded structural characterization by conventional approaches.
Figures



References
-
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. - PubMed
-
- Ames JB, Tanaka T, Ikura M, Stryer L. Nuclear magnetic resonance evidence for Ca(2+)-induced extrusion of the myristoyl group of recoverin. J Biol Chem. 1995;270:30909–30913. - PubMed
-
- Ames JB, Tanaka T, Stryer L, Ikura M. Secondary structure of myristoylated recoverin determined by three-dimensional heteronuclear NMR: implications for the calcium-myristoyl switch. Biochemistry. 1994;33:10743–10753. - PubMed
-
- Babu CR, Flynn PF, Wand AJ. Preparation, characterization, and NMR spectroscopy of encapsulated proteins dissolved in low viscosity fluids. J Biomol NMR. 2003;25:313–323. - PubMed
-
- Babu CR, Hilser VJ, Wand AJ. Direct access to the cooperative substructure of proteins and the protein ensemble via cold denaturation. Nat Struct Mol Biol. 2004;11:352–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

