Local protease signaling contributes to neural tube closure in the mouse embryo
- PMID: 20152175
- PMCID: PMC2822780
- DOI: 10.1016/j.devcel.2009.11.014
Local protease signaling contributes to neural tube closure in the mouse embryo
Abstract
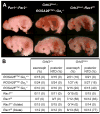
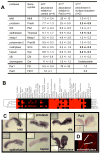
We report an unexpected role for protease signaling in neural tube closure and the formation of the central nervous system. Mouse embryos lacking protease-activated receptors 1 and 2 showed defective hindbrain and posterior neuropore closure and developed exencephaly and spina bifida, important human congenital anomalies. Par1 and Par2 were expressed in surface ectoderm, and Par2 was expressed selectively along the line of closure. Ablation of G(i/z) and Rac1 function in these Par2-expressing cells disrupted neural tube closure, further implicating G protein-coupled receptors and identifying a likely effector pathway. Cluster analysis of protease and Par2 expression patterns revealed a group of membrane-tethered proteases often coexpressed with Par2. Among these, matriptase activated Par2 with picomolar potency, and hepsin and prostasin activated matriptase. Together, our results suggest a role for protease-activated receptor signaling in neural tube closure and identify a local protease network that may trigger Par2 signaling and monitor and regulate epithelial integrity in this context.
(c) 2010 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Defining a PARticular pathway of neural tube closure.Dev Cell. 2010 Jan 19;18(1):1-2. doi: 10.1016/j.devcel.2010.01.002. Dev Cell. 2010. PMID: 20152170
References
-
- Auden A, Caddy J, Wilanowski T, Ting SB, Cunningham JM, Jane SM. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr Patterns. 2006;6:964–970. - PubMed
-
- Becker RC, Moliterno DJ, Jennings LK, Pieper KS, Pei J, Niederman A, Ziada KM, Berman G, Strony J, Joseph D, et al. Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet. 2009;373:919–928. - PubMed
-
- Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem. 2001;268:1439–1447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

