Tenectin is a novel alphaPS2betaPS integrin ligand required for wing morphogenesis and male genital looping in Drosophila
- PMID: 20152825
- PMCID: PMC2854234
- DOI: 10.1016/j.ydbio.2010.02.008
Tenectin is a novel alphaPS2betaPS integrin ligand required for wing morphogenesis and male genital looping in Drosophila
Abstract
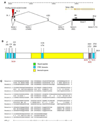
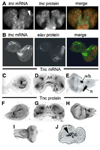
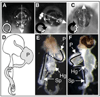
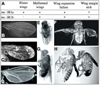
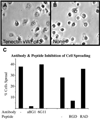
Morphogenesis of the adult structures of holometabolous insects is regulated by ecdysteroids and juvenile hormones and involves cell-cell interactions mediated in part by the cell surface integrin receptors and their extracellular matrix (ECM) ligands. These adhesion molecules and their regulation by hormones are not well characterized. We describe the gene structure of a newly described ECM molecule, tenectin, and demonstrate that it is a hormonally regulated ECM protein required for proper morphogenesis of the adult wing and male genitalia. Tenectin's function as a new ligand of the PS2 integrins is demonstrated by both genetic interactions in the fly and by cell spreading and cell adhesion assays in cultured cells. Its interaction with the PS2 integrins is dependent on RGD and RGD-like motifs. Tenectin's function in looping morphogenesis in the development of the male genitalia led to experiments that demonstrate a role for PS integrins in the execution of left-right asymmetry.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Role of the PS integrins in Drosophila development.Immunol Cell Biol. 1995 Dec;73(6):558-64. doi: 10.1038/icb.1995.89. Immunol Cell Biol. 1995. PMID: 8713479 Review.
-
The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila.Development. 1998 May;125(9):1679-89. doi: 10.1242/dev.125.9.1679. Development. 1998. PMID: 9521906
-
Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium.J Cell Sci. 2007 Mar 15;120(Pt 6):1061-71. doi: 10.1242/jcs.03404. Epub 2007 Feb 27. J Cell Sci. 2007. PMID: 17327274
-
Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2.J Biol Chem. 1998 Jul 17;273(29):18235-41. doi: 10.1074/jbc.273.29.18235. J Biol Chem. 1998. PMID: 9660786
-
The PS integrins are required for a regulatory event during Drosophila wing morphogenesis.Ann N Y Acad Sci. 1998 Oct 23;857:99-109. doi: 10.1111/j.1749-6632.1998.tb10110.x. Ann N Y Acad Sci. 1998. PMID: 9917835 Review.
Cited by
-
Genome-Wide Association Study of Circadian Behavior in Drosophila melanogaster.Behav Genet. 2019 Jan;49(1):60-82. doi: 10.1007/s10519-018-9932-0. Epub 2018 Oct 19. Behav Genet. 2019. PMID: 30341464 Free PMC article.
-
Tenectin recruits integrin to stabilize bouton architecture and regulate vesicle release at the Drosophila neuromuscular junction.Elife. 2018 Jun 14;7:e35518. doi: 10.7554/eLife.35518. Elife. 2018. PMID: 29901439 Free PMC article.
-
Transcriptome analysis reveals signature of adaptation to landscape fragmentation.PLoS One. 2014 Jul 2;9(7):e101467. doi: 10.1371/journal.pone.0101467. eCollection 2014. PLoS One. 2014. PMID: 24988207 Free PMC article.
-
Left-right asymmetric cell intercalation drives directional collective cell movement in epithelial morphogenesis.Nat Commun. 2015 Dec 10;6:10074. doi: 10.1038/ncomms10074. Nat Commun. 2015. PMID: 26656655 Free PMC article.
-
Hormone-dependent control of developmental timing through regulation of chromatin accessibility.Genes Dev. 2017 May 1;31(9):862-875. doi: 10.1101/gad.298182.117. Epub 2017 May 23. Genes Dev. 2017. PMID: 28536147 Free PMC article.
References
-
- Ádám G, Perrimon N, Noselli S. The retinoic-like juvenile hormone controls the looping of left-right asymmetric organs in Drosophila. Development. 2003;130:2397–2406. - PubMed
-
- Andres AJ, Fletcher JC, Karim FD, Thummel CS. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev. Biol. 1993;160:388–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

