Midline signaling regulates kidney positioning but not nephrogenesis through Shh
- PMID: 20152829
- PMCID: PMC2854326
- DOI: 10.1016/j.ydbio.2010.02.007
Midline signaling regulates kidney positioning but not nephrogenesis through Shh
Abstract
The role of axial structures, especially the notochord, in metanephric kidney development has not been directly examined. Here, we showed that disruption of the notochord and floor plate by diphtheria toxin (DTA)-mediated cell ablation did not disrupt nephrogenesis, but resulted in kidney fusions, resembling horseshoe kidneys in humans. Axial disruptions led to more medially positioned metanephric mesenchyme (MM) in midgestation. However, neither axial disruption nor the ensuing positional shift of the MM affected the formation of nephrons and other structures within the kidney. Response to Shh signaling was greatly reduced in midline cell populations in the mutants. To further ascertain the molecular mechanism underlying these abnormalities, we specifically inactivated Shh in the notochord and floor plate. We found that depleting the axial source of Shh was sufficient to cause kidney fusion, even in the presence of the notochord. These results suggested that the notochord is dispensable for nephrogenesis but required for the correct positioning of the metanephric kidney. Axial Shh signal appears to be critical in conferring the effects of axial structures on kidney positioning along the mediolateral axis. These studies also provide insights into the pathogenesis of horseshoe kidneys and how congenital kidney defects can be caused by signals outside the renal primordia.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

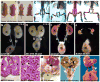
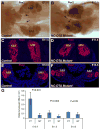



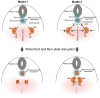
Similar articles
-
Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm.Dev Biol. 2014 Feb 1;386(1):216-26. doi: 10.1016/j.ydbio.2013.12.026. Epub 2013 Dec 24. Dev Biol. 2014. PMID: 24370450 Free PMC article.
-
Differential patterning of ventral midline cells by axial mesoderm is regulated by BMP7 and chordin.Development. 1999 Jan;126(2):397-408. doi: 10.1242/dev.126.2.397. Development. 1999. PMID: 9847252
-
Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways.Dev Biol. 2000 Mar 15;219(2):350-63. doi: 10.1006/dbio.1999.9589. Dev Biol. 2000. PMID: 10694427
-
The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail.Development. 1997 Jan;124(2):327-42. doi: 10.1242/dev.124.2.327. Development. 1997. PMID: 9053309
-
Lineage-specific roles of hedgehog-GLI signaling during mammalian kidney development.Pediatr Nephrol. 2020 May;35(5):725-731. doi: 10.1007/s00467-019-04240-8. Epub 2019 Mar 28. Pediatr Nephrol. 2020. PMID: 30923969 Review.
Cited by
-
A retrotransposon insertion in the 5' regulatory domain of Ptf1a results in ectopic gene expression and multiple congenital defects in Danforth's short tail mouse.PLoS Genet. 2013;9(2):e1003206. doi: 10.1371/journal.pgen.1003206. Epub 2013 Feb 21. PLoS Genet. 2013. PMID: 23437001 Free PMC article.
-
Impaired intermediate formation in mouse embryos expressing reduced levels of Tbx6.Genesis. 2019 Mar;57(3):e23270. doi: 10.1002/dvg.23270. Epub 2019 Jan 12. Genesis. 2019. PMID: 30548789 Free PMC article.
-
Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm.Dev Biol. 2014 Feb 1;386(1):216-26. doi: 10.1016/j.ydbio.2013.12.026. Epub 2013 Dec 24. Dev Biol. 2014. PMID: 24370450 Free PMC article.
-
The notochord: structure and functions.Cell Mol Life Sci. 2015 Aug;72(16):2989-3008. doi: 10.1007/s00018-015-1897-z. Epub 2015 Apr 2. Cell Mol Life Sci. 2015. PMID: 25833128 Free PMC article. Review.
-
An atypical basement membrane forms a midline barrier during left-right asymmetric gut development in the chicken embryo.Elife. 2025 Apr 29;12:RP89494. doi: 10.7554/eLife.89494. Elife. 2025. PMID: 40298919 Free PMC article.
References
-
- Aoki Y, Mori S, Kitajima K, Yokoyama O, Kanamaru H, Okada K, Yokota Y. Id2 haploinsufficiency in mice leads to congenital hydronephrosis resembling that in humans. Genes Cells. 2004;9:1287–96. - PubMed
-
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. - PubMed
-
- Barak H, Rosenfelder L, Schultheiss TM, Reshef R. Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev Dyn. 2005;232:901–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

