Fluorescence Detection of Heavy Atom Labeling (FD-HAL): a rapid method for identifying covalently modified cysteine residues by phasing atoms
- PMID: 20152903
- PMCID: PMC12042814
- DOI: 10.1016/j.jsb.2010.02.005
Fluorescence Detection of Heavy Atom Labeling (FD-HAL): a rapid method for identifying covalently modified cysteine residues by phasing atoms
Abstract
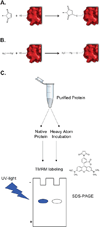
Membrane protein crystallography frequently stalls at the phase determination stage due to poor crystal diffraction and the inability to identify heavy atom derivatization prior to data collection. Thus, a majority of time, effort and resources are invested preparing potential derivatized crystals for synchrotron data collection and analysis without knowledge of heavy atom labeling. To remove this uncertainty, we introduce Fluorescence Detection of Heavy Atom Labeling (FD-HAL) using tetramethylrhodamine-5-maleimide (a fluorescent maleimide compound) to monitor in-gel cysteine residue accessibility and ascertain covalent modification by mercury, platinum and gold compounds. We have tested this technique on three integral membrane proteins (LacY, vSGLT and mVDAC1) and can quickly assess the optimal concentrations, time and heavy atom compound to derivatize free cysteine residues in order to facilitate crystal phasing. This, in conjunction with cysteine scanning for incorporating heavy atoms at strategic positions, is a useful tool that will considerably assist in phasing membrane protein structures.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- White SH. Biophysical dissection of membrane proteins. Nature. 2009 May 21;459(7245):344–346. - PubMed
-
- Boggon TJ, Shapiro L. Screening for phasing atoms in protein crystallography. Structure. 2000;Vol 8(No 7):R143–R149. - PubMed
-
- Cohen SL, Padovan JC, Chait BT. Mass spectrometric analysis of mercury incorporation into proteins for X-ray diffraction phase determination. Anal Chem. 2000 Feb 1;72(3):574–579. - PubMed
-
- Sun PD, Hammer CH. Mass-spectrometry assisted heavy-atom derivative screening of human Fc gamma RIII crystals. Acta Crystallogr D Biol Crystallogr. 2000 Feb;56(Pt 2):161–168. - PubMed
-
- Wittig I, Schägger H. Features and applications of blue-native and clear-native electrophoresis. Proteomics. 2008 Oct;8(19):3974–3990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

