Inhibition of LINE-1 and Alu retrotransposition by exosomes encapsidating APOBEC3G and APOBEC3F
- PMID: 20153011
- PMCID: PMC2851184
- DOI: 10.1016/j.virol.2010.01.021
Inhibition of LINE-1 and Alu retrotransposition by exosomes encapsidating APOBEC3G and APOBEC3F
Abstract
Human cytidine deaminases, including APOBEC3G (A3G) and A3F, are part of a cellular defense system against retroviruses and retroelements including non-LTR retrotransposons LINE-1 (L1) and Alu. Expression of cellular A3 proteins is sufficient for inhibition of L1 and Alu retrotransposition, but the effect of A3 proteins transferred in exosomes on retroelement mobilization is unknown. Here, we demonstrate for the first time that exosomes secreted by CD4(+)H9 T cells and mature monocyte-derived dendritic cells encapsidate A3G and A3F and inhibit L1 and Alu retrotransposition. A3G is the major contributor to the inhibitory activity of exosomes, however, the contribution of A3F in H9 exosomes cannot be excluded. Additionally, we show that exosomes encapsidate mRNAs coding for A3 proteins. A3G mRNA, and less so A3F, was enriched in exosomes secreted by H9 cells. Exosomal A3G mRNA was functional in vitro. Whether exosomes inhibit retrotransposons in vivo requires further investigation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
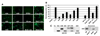
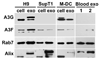

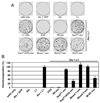
Similar articles
-
Selective inhibition of Alu retrotransposition by APOBEC3G.Gene. 2007 Apr 1;390(1-2):199-205. doi: 10.1016/j.gene.2006.08.032. Epub 2006 Sep 27. Gene. 2007. PMID: 17079095 Free PMC article.
-
Comparison of cellular ribonucleoprotein complexes associated with the APOBEC3F and APOBEC3G antiviral proteins.J Virol. 2008 Jun;82(11):5636-42. doi: 10.1128/JVI.00287-08. Epub 2008 Mar 26. J Virol. 2008. PMID: 18367521 Free PMC article.
-
High-molecular-mass APOBEC3G complexes restrict Alu retrotransposition.Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15588-93. doi: 10.1073/pnas.0604524103. Epub 2006 Oct 9. Proc Natl Acad Sci U S A. 2006. PMID: 17030807 Free PMC article.
-
APOBEC3G: an intracellular centurion.Philos Trans R Soc Lond B Biol Sci. 2009 Mar 12;364(1517):689-703. doi: 10.1098/rstb.2008.0193. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19008196 Free PMC article. Review.
-
Post-transcriptional regulation of LINE-1 retrotransposition by AID/APOBEC and ADAR deaminases.Chromosome Res. 2018 Mar;26(1-2):45-59. doi: 10.1007/s10577-018-9572-5. Epub 2018 Feb 2. Chromosome Res. 2018. PMID: 29396793 Review.
Cited by
-
Footprint of APOBEC3 on the genome of human retroelements.J Virol. 2013 Jul;87(14):8195-204. doi: 10.1128/JVI.00298-13. Epub 2013 May 22. J Virol. 2013. PMID: 23698293 Free PMC article.
-
Increased expression with differential subcellular location of cytidine deaminase APOBEC3G in human CD4(+) T-cell activation and dendritic cell maturation.Immunol Cell Biol. 2016 Aug;94(7):689-700. doi: 10.1038/icb.2016.28. Epub 2016 Mar 18. Immunol Cell Biol. 2016. PMID: 26987686
-
The evidence for increased L1 activity in the site of human adult brain neurogenesis.PLoS One. 2015 Feb 17;10(2):e0117854. doi: 10.1371/journal.pone.0117854. eCollection 2015. PLoS One. 2015. PMID: 25689626 Free PMC article.
-
Molecular model linking Th2 polarized M2 tumour-associated macrophages with deaminase-mediated cancer progression mutation signatures.Scand J Immunol. 2019 May;89(5):e12760. doi: 10.1111/sji.12760. Epub 2019 Mar 18. Scand J Immunol. 2019. PMID: 30802996 Free PMC article.
-
Suppression of APOBEC3-mediated restriction of HIV-1 by Vif.Front Microbiol. 2014 Aug 26;5:450. doi: 10.3389/fmicb.2014.00450. eCollection 2014. Front Microbiol. 2014. PMID: 25206352 Free PMC article. Review.
References
-
- Alce TM, Popik W. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J Biol Chem. 2004;279(33):34083–34086. - PubMed
-
- Anant S, Davidson NO. Hydrolytic nucleoside and nucleotide deamination, and genetic instability: a possible link between RNA-editing enzymes and cancer? Trends Mol Med. 2003;9(4):147–152. - PubMed
-
- Babushok DV, Kazazian HH., Jr Progress in understanding the biology of the human mutagen LINE-1. Hum Mutat. 2007;28(6):527–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

