Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes
- PMID: 20153045
- PMCID: PMC2837121
- DOI: 10.1016/j.biomaterials.2010.01.068
Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes
Abstract
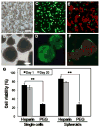
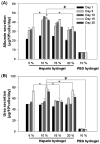
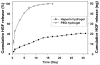
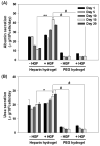
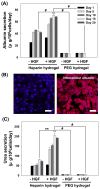
Primary hepatocytes are commonly used as liver surrogates in toxicology and tissue engineering fields, therefore, maintenance of functional hepatocytes in vitro is an important topic of investigation. This paper sought to characterize heparin-based hydrogel as a three-dimensional scaffold for hepatocyte culture. The primary rat hepatocytes were mixed with a prepolymer solution comprised of thiolated heparin and acrylated poly(ethylene glycol) (PEG). Raising the temperature from 25 degrees to 37 degrees C initiated Michael addition reaction between the thiol and acrylated moieties and resulted in formation of hydrogel with entrapped cells. Analysis of liver-specific products, albumin and urea, revealed that the heparin hydrogel was non-cytotoxic to cells and, in fact, promoted hepatic function. Hepatocytes entrapped in the heparin-based hydrogel maintained high levels of albumin and urea synthesis after three weeks in culture. Because heparin is known to bind growth factors, we incorporated hepatocyte growth factor (HGF)-an important liver signaling molecule - into the hydrogel. HGF release from heparin hydrogel matrix was analyzed using enzyme linked immunoassay (ELISA) and was shown to occur in a controlled manner with only 40% of GF molecules released after 30 days in culture. Importantly, hepatocytes cultured within HGF-containing hydrogels exhibited significantly higher levels of albumin and urea synthesis compared to cells cultured in the hydrogel alone. Overall, heparin-based hydrogel showed to be a promising matrix for encapsulation and maintenance of difficult-to-culture primary hepatocytes. In the future, we envision employing heparin-based hyrogels as matrices for in vitro differentiation of hepatocytes or stem cells and as vehicles for transplantation of these cells.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
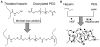
Similar articles
-
Multilayered heparin hydrogel microwells for cultivation of primary hepatocytes.Adv Healthc Mater. 2014 Jan;3(1):126-32. doi: 10.1002/adhm.201300054. Epub 2013 Jul 5. Adv Healthc Mater. 2014. PMID: 23828859 Free PMC article.
-
Impact of Nanotopography, Heparin Hydrogel Microstructures, and Encapsulated Fibroblasts on Phenotype of Primary Hepatocytes.ACS Appl Mater Interfaces. 2015 Jun 17;7(23):12299-308. doi: 10.1021/am504614e. Epub 2014 Sep 23. ACS Appl Mater Interfaces. 2015. PMID: 25247391 Free PMC article.
-
Characterizing the effects of heparin gel stiffness on function of primary hepatocytes.Tissue Eng Part A. 2013 Dec;19(23-24):2655-63. doi: 10.1089/ten.TEA.2012.0681. Epub 2013 Aug 16. Tissue Eng Part A. 2013. PMID: 23815179 Free PMC article.
-
Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering.Biomacromolecules. 2014 Jan 13;15(1):206-18. doi: 10.1021/bm4015039. Epub 2013 Dec 23. Biomacromolecules. 2014. PMID: 24350561
-
Recent Developments in Thiolated Polymeric Hydrogels for Tissue Engineering Applications.Tissue Eng Part B Rev. 2018 Feb;24(1):66-74. doi: 10.1089/ten.TEB.2016.0442. Epub 2017 Aug 24. Tissue Eng Part B Rev. 2018. PMID: 28726576 Review.
Cited by
-
Influence of 3D porous galactose containing PVA/gelatin hydrogel scaffolds on three-dimensional spheroidal morphology of hepatocytes.J Mater Sci Mater Med. 2015 Jan;26(1):5345. doi: 10.1007/s10856-014-5345-7. Epub 2015 Jan 13. J Mater Sci Mater Med. 2015. PMID: 25578699
-
Modeling iontophoretic drug delivery in a microfluidic device.Lab Chip. 2020 Sep 21;20(18):3310-3321. doi: 10.1039/d0lc00602e. Epub 2020 Sep 1. Lab Chip. 2020. PMID: 32869052 Free PMC article.
-
Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications.Acta Biomater. 2014 Apr;10(4):1588-600. doi: 10.1016/j.actbio.2013.07.031. Epub 2013 Aug 2. Acta Biomater. 2014. PMID: 23911941 Free PMC article. Review.
-
Bioactive hydrogel microcapsules for guiding stem cell fate decisions by release and reloading of growth factors.Bioact Mater. 2021 Dec 20;15:1-14. doi: 10.1016/j.bioactmat.2021.12.008. eCollection 2022 Sep. Bioact Mater. 2021. PMID: 35386345 Free PMC article.
-
The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin.Biomaterials. 2011 Mar;32(8):2059-69. doi: 10.1016/j.biomaterials.2010.11.038. Epub 2010 Dec 13. Biomaterials. 2011. PMID: 21147501 Free PMC article.
References
-
- Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13(7):880–5. - PubMed
-
- Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, Yarmush ML. Hepatic tissue engineering for adjunct and temporary liver support: Critical technologies. Liver Transplant. 2004;10(11):1331–42. - PubMed
-
- Allen JW, Bhatia SN. Engineering liver therapies for the future. Tissue Eng. 2002;8(5):725–37. - PubMed
-
- Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuartion. FASEB J. 1996:101471–84. - PubMed
-
- Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes - Control by extracellular-matrix. J Cell Physiol. 1992;151(3):497–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

