Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84
- PMID: 20153220
- PMCID: PMC2830297
- DOI: 10.1016/j.immuni.2010.01.010
Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84
Abstract
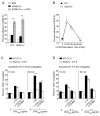
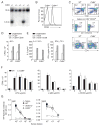
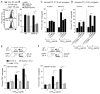
CD4(+) T cells deficient in signaling lymphocyte activation molecule (SLAM)-associated protein (SAP) exhibit a selective impairment in adhesion to antigen-presenting B cells but not dendritic cells (DCs), resulting in defective germinal center formation. However, the nature of this selective adhesion defect remained unclear. We found that whereas T cell:DC interactions were primarily integrin dependent, T cell:B cell interactions had both an early integrin-dependent phase and a sustained phase that also required SAP. We further found that the SLAM family member CD84 was required for prolonged T cell:B cell contact, optimal T follicular helper function, and germinal center formation in vivo. Moreover, both CD84 and another SLAM member, Ly108, mediated T cell adhesion and participated in stable T cell:B cell interactions in vitro. Our results reveal insight into the dynamic regulation of T cell:B cell interactions and identify SLAM family members as critical components of sustained T cell:B cell adhesion required for productive humoral immunity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
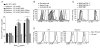



References
-
- Al-Alwan MM, Rowden G, Lee TDG, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. - PubMed
-
- Barber DF, Long EO. Coexpression of CD58 or CD48 with intracellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J Immunol. 2003;170:294–299. - PubMed
-
- Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VLJ, Amigorena S. Requirements of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

