Design and characterization of a constitutively open KcsA
- PMID: 20153331
- PMCID: PMC2848999
- DOI: 10.1016/j.febslet.2010.02.015
Design and characterization of a constitutively open KcsA
Abstract
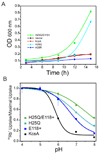
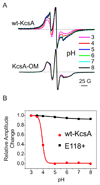
The molecular nature of the structure responsible for proton sensitivity in KcsA has been identified as a charge cluster that surrounds the inner helical bundle gate. Here, we show that this proton sensor can be modified to engineer a constitutively open form of KcsA, amenable to functional, spectroscopic and structural analyses. By combining charge neutralizations for all acidic and basic residues in the cluster at positions 25, 117-122 and 124 (but not E118), a mutant KcsA is generated that displays constitutively open channel activity up to pH 9. The structure of this mutant revealed that full opening appears to be inhibited by lattice forces since the activation gate seems to be only on the early stages of opening.
Copyright 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Mechanism of activation gating in the full-length KcsA K+ channel.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11896-9. doi: 10.1073/pnas.1105112108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730186 Free PMC article.
-
A molecular mechanism for proton-dependent gating in KcsA.FEBS Lett. 2010 Mar 19;584(6):1126-32. doi: 10.1016/j.febslet.2010.02.003. Epub 2010 Feb 6. FEBS Lett. 2010. PMID: 20138880 Free PMC article.
-
Investigation of a KcsA Cytoplasmic pH Gate in Lipoprotein Nanodiscs.Chembiochem. 2019 Mar 15;20(6):813-821. doi: 10.1002/cbic.201800627. Epub 2019 Feb 5. Chembiochem. 2019. PMID: 30565824
-
Molecular determinants of gating at the potassium-channel selectivity filter.Nat Struct Mol Biol. 2006 Apr;13(4):311-8. doi: 10.1038/nsmb1069. Epub 2006 Mar 12. Nat Struct Mol Biol. 2006. PMID: 16532009
-
Potassium channels.FEBS Lett. 2003 Nov 27;555(1):62-5. doi: 10.1016/s0014-5793(03)01104-9. FEBS Lett. 2003. PMID: 14630320 Review.
Cited by
-
Full opening of helix bundle crossing does not lead to NaK channel activation.J Gen Physiol. 2022 Dec 5;154(12):e202213196. doi: 10.1085/jgp.202213196. Epub 2022 Nov 3. J Gen Physiol. 2022. PMID: 36326620 Free PMC article.
-
Role of the KcsA channel cytoplasmic domain in pH-dependent gating.Biophys J. 2011 Nov 2;101(9):2157-62. doi: 10.1016/j.bpj.2011.09.024. Epub 2011 Nov 1. Biophys J. 2011. PMID: 22067153 Free PMC article.
-
The Hydrophobic Effect Contributes to the Closed State of a Simplified Ion Channel through a Conserved Hydrophobic Patch at the Pore-Helix Crossing.Front Pharmacol. 2015 Nov 27;6:284. doi: 10.3389/fphar.2015.00284. eCollection 2015. Front Pharmacol. 2015. PMID: 26640439 Free PMC article.
-
The influence of membrane bilayer thickness on KcsA channel activity.Channels (Austin). 2019 Dec;13(1):424-439. doi: 10.1080/19336950.2019.1676367. Channels (Austin). 2019. PMID: 31608774 Free PMC article.
-
Mechanism of activation gating in the full-length KcsA K+ channel.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11896-9. doi: 10.1073/pnas.1105112108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730186 Free PMC article.
References
-
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
-
- Holmgren M, Shin KS, Yellen G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 1998;21:617–621. - PubMed
-
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. - PubMed
-
- Perozo E, Cortes DM, Cuello LG. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat Struct Biol. 1998;5:459–469. - PubMed
-
- Perozo E, Cortes DM, Cuello LG. Structural rearrangements underlying K+- channel activation gating. Science. 1999;285:73–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

