Critical brain circuits at the intersection between stress and learning
- PMID: 20153364
- PMCID: PMC2900534
- DOI: 10.1016/j.neubiorev.2010.02.002
Critical brain circuits at the intersection between stress and learning
Abstract
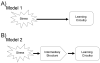
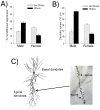
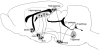
The effects of stressful life experience on learning are pervasive and vary greatly both within and between individuals. It is therefore unlikely that any one mechanism will underlie these complicated processes. Nonetheless, without identifying the necessary and sufficient circuitry, no complete mechanism or set of mechanisms can be identified. In this review, we provide two anatomical frameworks through which stressful life experience can influence processes related to learning and memory. In the first, stressful experience releases stress hormones, primarily from the adrenals, which directly impact brain areas engaged in learning. In the second, stressful experience indirectly alters the circuits used in learning via intermediary brain regions. Importantly, these intermediary brain regions are not integral to the stress response or learning itself, but rather link the consequences of a stressful experience with circuits used to learn associations. As reviewed, the existing literature provides support for both frameworks, with somewhat more support for the first but sufficient evidence for the latter which involves intermediary structures. Once we determine the circumstances that engage each framework and identify which one is most predominant, we can begin to focus our efforts on describing the neuronal and hormonal mechanisms that operate within these circuits to influence cognitive processes after stressful life experience.
Figures
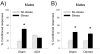
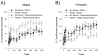
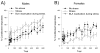
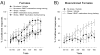
References
-
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. - PubMed
-
- Aggleton JP, Friedman DP, Mishkin M. A comparison between the connections of the amygdala and hippocampus with the basal forebrain in the macaque. Experimental Brain Research. 1987;67(3):556–68. - PubMed
-
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances.[see comment]. [Review] [81 refs] Hippocampus. 2001;11(1):8–17. - PubMed
-
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. - PubMed
-
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. [References] British Journal of Psychiatry. 2001;178:206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

