DmsD, a Tat system specific chaperone, interacts with other general chaperones and proteins involved in the molybdenum cofactor biosynthesis
- PMID: 20153451
- PMCID: PMC3288112
- DOI: 10.1016/j.bbapap.2010.01.022
DmsD, a Tat system specific chaperone, interacts with other general chaperones and proteins involved in the molybdenum cofactor biosynthesis
Abstract
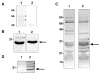
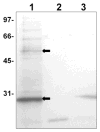
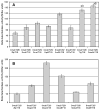
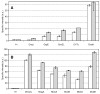
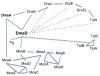
Many bacterial oxidoreductases depend on the Tat translocase for correct cell localization. Substrates for the Tat translocase possess twin-arginine leaders. System specific chaperones or redox enzyme maturation proteins (REMPs) are a group of proteins implicated in oxidoreductase maturation. DmsD is a REMP discovered in Escherichia coli, which interacts with the twin-arginine leader sequence of DmsA, the catalytic subunit of DMSO reductase. In this study, we identified several potential interacting partners of DmsD by using several in vitro protein-protein interaction screening approaches, including affinity chromatography, co-precipitation, and cross-linking. Candidate hits from these in vitro findings were analyzed by in vivo methods of bacterial two-hybrid (BACTH) and bimolecular fluorescence complementation (BiFC). From these data, DmsD was confirmed to interact with the general molecular chaperones DnaK, DnaJ, GrpE, GroEL, Tig and Ef-Tu. In addition, DmsD was also found to interact with proteins involved in the molybdenum cofactor biosynthesis pathway. Our data suggests that DmsD may play a role as a "node" in escorting its substrate through a cascade of chaperone assisted protein-folding maturation events.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
The hydrophobic region of the DmsA twin-arginine leader peptide determines specificity with chaperone DmsD.Biochemistry. 2013 Oct 29;52(43):7532-41. doi: 10.1021/bi4009374. Epub 2013 Oct 21. Biochemistry. 2013. PMID: 24093457 Free PMC article.
-
Visualizing interactions along the Escherichia coli twin-arginine translocation pathway using protein fragment complementation.PLoS One. 2010 Feb 16;5(2):e9225. doi: 10.1371/journal.pone.0009225. PLoS One. 2010. PMID: 20169075 Free PMC article.
-
Identification of protein-protein interactions between the TatB and TatC subunits of the twin-arginine translocase system and respiratory enzyme specific chaperones.Biochim Biophys Acta. 2016 Apr;1858(4):767-75. doi: 10.1016/j.bbamem.2016.01.025. Epub 2016 Jan 28. Biochim Biophys Acta. 2016. PMID: 26826271
-
Assembly pathway of a bacterial complex iron sulfur molybdoenzyme.Biomol Concepts. 2017 Sep 26;8(3-4):155-167. doi: 10.1515/bmc-2017-0011. Biomol Concepts. 2017. PMID: 28688222 Review.
-
The role of FeS clusters for molybdenum cofactor biosynthesis and molybdoenzymes in bacteria.Biochim Biophys Acta. 2015 Jun;1853(6):1335-49. doi: 10.1016/j.bbamcr.2014.09.021. Epub 2014 Sep 28. Biochim Biophys Acta. 2015. PMID: 25268953 Free PMC article. Review.
Cited by
-
History of Maturation of Prokaryotic Molybdoenzymes-A Personal View.Molecules. 2023 Oct 20;28(20):7195. doi: 10.3390/molecules28207195. Molecules. 2023. PMID: 37894674 Free PMC article. Review.
-
The biogenesis of β-lactamase enzymes.Microbiology (Reading). 2022 Aug;168(8):001217. doi: 10.1099/mic.0.001217. Microbiology (Reading). 2022. PMID: 35943884 Free PMC article. Review.
-
Chaperones in maturation of molybdoenzymes: Why specific is better than general?Bioengineered. 2017 Mar 4;8(2):133-136. doi: 10.1080/21655979.2016.1218579. Epub 2016 Aug 31. Bioengineered. 2017. PMID: 27580420 Free PMC article.
-
NarJ subfamily system specific chaperone diversity and evolution is directed by respiratory enzyme associations.BMC Evol Biol. 2015 Jun 12;15:110. doi: 10.1186/s12862-015-0412-3. BMC Evol Biol. 2015. PMID: 26067063 Free PMC article.
-
Biosynthesis and Insertion of the Molybdenum Cofactor.EcoSal Plus. 2015;6(2):10.1128/ecosalplus.ESP-0006-2013. doi: 10.1128/ecosalplus.ESP-0006-2013. EcoSal Plus. 2015. PMID: 26435257 Free PMC article. Review.
References
-
- Berks BC. A common export pathway for proteins binding complex redox cofactors. Mol Microbiol. 1996;22:393–404. - PubMed
-
- Weiner JH, Bilous PT, Shaw GM, Lubitz SP, Frost L, Thomas GH, Cole JA, Turner RJ. A novel and ubiquitous system for membrane targeting and secretion of proteins in the folded state. Cell. 1998;93:93–101. - PubMed
-
- Berks BC, Sargent F, Palmer T. The Tat protein export pathway. Mol Microbiol. 2000;35:260–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

