Topology of the membrane-bound form of complement protein C9 probed by glycosylation mapping, anti-peptide antibody binding, and disulfide modification
- PMID: 20153530
- PMCID: PMC2849895
- DOI: 10.1016/j.molimm.2010.01.013
Topology of the membrane-bound form of complement protein C9 probed by glycosylation mapping, anti-peptide antibody binding, and disulfide modification
Abstract
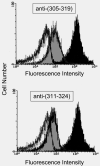
The two N-linked oligosaccharides in native human C9 were deleted by site-specific mutagenesis. This aglycosyl-C9 did not differ from its native form in hemolytic and bactericidal activity. A new N-glycosylation site (K311N/E313T) was introduced into the turn of a helix-turn-helix [HTH] fold that had been postulated to form a transmembrane hairpin in membrane-bound C9. This glycosylated form of human C9 was as active as the native protein suggesting that the glycan chain remains on the external side of the membrane and that translocation of this hairpin is not required for membrane anchoring. Furthermore, flow cytometry provided evidence for the recognition of membrane-bound C9 on complement-lysed ghosts by an antibody specific for the HTH fold. A new N-glycosylation site (P26N) was also introduced close to the N-terminus of C9 to test whether this region was involved in C9 polymerization, which is thought to be required for cytolytic activity of C9. Again, this glycosylated C9 was as active as native C9 and could be induced to polymerize by heating or incubation with metal ions. The two C-terminal cystines within the MACPF domain could be eliminated partially or completely without affecting the hemolytic activity. Free sulfhydryl groups of unpaired cysteines in such C9 mutants are blocked since they could not be modified with SH-specific reagents. These results are discussed with respect to a recently proposed model that, on the basis of the MACPF structure in C8alpha, envisions membrane insertion of C9 to resemble the mechanism by which cholesterol-dependent cytolysins enter a membrane.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Membrane pore formation by human complement: functional importance of the transmembrane β-hairpin (TMH) segments of C8α and C9.Mol Immunol. 2014 Feb;57(2):310-6. doi: 10.1016/j.molimm.2013.10.007. Epub 2013 Nov 12. Mol Immunol. 2014. PMID: 24239861 Free PMC article.
-
Binding of human complement C8 to C9: role of the N-terminal modules in the C8 alpha subunit.Biochemistry. 2002 Dec 10;41(49):14546-51. doi: 10.1021/bi026641j. Biochemistry. 2002. PMID: 12463754
-
Functional studies of the MACPF domain of human complement protein C8alpha reveal sites for simultaneous binding of C8beta, C8gamma, and C9.Biochemistry. 2006 Apr 25;45(16):5290-6. doi: 10.1021/bi0601860. Biochemistry. 2006. PMID: 16618117
-
Transmembrane channel-formation by five complement proteins.Biochem Soc Symp. 1985;50:235-46. Biochem Soc Symp. 1985. PMID: 2428370 Review.
-
The killer molecule of complement.J Invest Dermatol. 1985 Jul;85(1 Suppl):47s-52s. doi: 10.1111/1523-1747.ep12275445. J Invest Dermatol. 1985. PMID: 3891882 Review.
Cited by
-
The Membrane Attack Complex/Perforin Superfamily.J Mol Microbiol Biotechnol. 2017;27(4):252-267. doi: 10.1159/000481286. Epub 2017 Nov 17. J Mol Microbiol Biotechnol. 2017. PMID: 29145176 Free PMC article.
-
Trichinella spiralis paramyosin binds to C8 and C9 and protects the tissue-dwelling nematode from being attacked by host complement.PLoS Negl Trop Dis. 2011 Jul;5(7):e1225. doi: 10.1371/journal.pntd.0001225. Epub 2011 Jul 5. PLoS Negl Trop Dis. 2011. PMID: 21750743 Free PMC article.
-
Comparative Genomics Reveal That Host-Innate Immune Responses Influence the Clinical Prevalence of Legionella pneumophila Serogroups.PLoS One. 2013 Jun 27;8(6):e67298. doi: 10.1371/journal.pone.0067298. Print 2013. PLoS One. 2013. PMID: 23826259 Free PMC article.
-
Membrane pore formation by human complement: functional importance of the transmembrane β-hairpin (TMH) segments of C8α and C9.Mol Immunol. 2014 Feb;57(2):310-6. doi: 10.1016/j.molimm.2013.10.007. Epub 2013 Nov 12. Mol Immunol. 2014. PMID: 24239861 Free PMC article.
-
Cardiac extracellular proteome profiling and membrane topology analysis using glycoproteomics.Proteomics Clin Appl. 2014 Aug;8(7-8):595-602. doi: 10.1002/prca.201400009. Proteomics Clin Appl. 2014. PMID: 24920555 Free PMC article.
References
-
- Amiguet P, Brunner J, Tschopp J. The membrane attack complex of complement: lipid insertion of tubular and nontubular polymerized C9. Biochemistry. 1985;24:7328–34. - PubMed
-
- Chang CP, Husler T, Zhao J, Wiedmer T, Sims PJ. Identity of a peptide domain of human C9 that is bound by the cell-surface complement inhibitor, CD59. J Biol Chem. 1994;269:26424–30. - PubMed
-
- Chen Q, Lord ST, Lentz BR. Construction, properties and specific fluorescent labeling of a bovine prothrombin mutant engineered with a free C-terminal cysteine. Protein Eng. 1996;9:545–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

