Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells
- PMID: 20153802
- PMCID: PMC2884222
- DOI: 10.1016/j.exphem.2010.02.002
Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells
Abstract
Objective: Having previously demonstrated that the complement system modulates mobilization of hematopoietic stem/progenitor cells (HSPC) in mice, we investigated the involvement of C5 cleavage fragments (C5a/(desArg)C5a) in human HSPC mobilization.
Materials and methods: C5 cleavage fragments in the plasma were evaluated by enzyme-linked immunosorbent assay using human anti-(desArg)C5a antibody, and expression of the C5a/(desArg)C5a receptor (CD88) in hematopoietic cells by flow cytometry. We also examined the chemotactic responses of hematopoietic cells to C5 cleavage fragments and expression of stromal cell-derived factor-1 (SDF-1)-degrading proteases that perturb retention of HSPC in bone marrow, namely matrix metalloproteinase (MMP)-9, membrane type (MT) 1-MMP, and carboxypeptidase M.
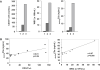
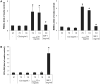
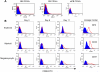
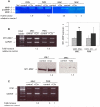
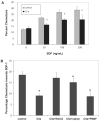
Results: We found that plasma levels of (desArg)C5a are significantly higher in patients who are good mobilizers and correlate with CD34(+) cell and white blood cell counts in mobilized peripheral blood. C5 cleavage fragments did not chemoattract myeloid progenitors (colony-forming unit granulocyte-macrophage), but (desArg)C5a did strongly chemoattract mature nucleated cells. Consistently, CD88 was not detected on CD34(+) cells, but appeared on more mature myeloid precursors, monocytes, and granulocytes. Moreover, granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells and polymorphonuclear cells had a significantly higher percentage of cells expressing CD88 than nonmobilized peripheral blood. Furthermore, C5a stimulation of granulocytes and monocytes decreased CXCR4 expression and chemotaxis toward an SDF-1 gradient and increased secretion of MMP-9 and expression of MT1-MMP and carboxypeptidase M.
Conclusion: C5 cleavage fragments not only induce a highly proteolytic microenvironment in human bone marrow, which perturbs retention through the CXCR4/SDF-1 axis, but also strongly chemoattracts granulocytes, promoting their egress into mobilized peripheral blood, which is crucial for subsequent mobilization of HSPC.
Figures



Similar articles
-
Signaling of the Complement Cleavage Product Anaphylatoxin C5a Through C5aR (CD88) Contributes to Pharmacological Hematopoietic Stem Cell Mobilization.Stem Cell Rev Rep. 2017 Dec;13(6):793-800. doi: 10.1007/s12015-017-9769-6. Stem Cell Rev Rep. 2017. PMID: 28918528 Free PMC article.
-
Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes.Leukemia. 2009 Nov;23(11):2052-62. doi: 10.1038/leu.2009.158. Epub 2009 Aug 6. Leukemia. 2009. PMID: 19657368 Free PMC article.
-
Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex.Leukemia. 2010 May;24(5):976-85. doi: 10.1038/leu.2010.53. Epub 2010 Apr 1. Leukemia. 2010. PMID: 20357827 Free PMC article.
-
Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells.Arch Immunol Ther Exp (Warsz). 2009 Jul-Aug;57(4):269-78. doi: 10.1007/s00005-009-0037-6. Epub 2009 Jul 4. Arch Immunol Ther Exp (Warsz). 2009. PMID: 19578812 Review.
-
A pivotal role of activation of complement cascade (CC) in mobilization of hematopoietic stem/progenitor cells (HSPC).Adv Exp Med Biol. 2008;632:47-60. Adv Exp Med Biol. 2008. PMID: 19025113 Review.
Cited by
-
Effect of Helicobacter pylori infection on stromal-derived factor-1/CXCR4 axis in bone marrow-derived mesenchymal stem cells.Adv Biomed Res. 2014 Jan 9;3:19. doi: 10.4103/2277-9175.124650. eCollection 2014. Adv Biomed Res. 2014. PMID: 24592369 Free PMC article.
-
Role of Neurotransmitters in Steady State Hematopoiesis, Aging, and Leukemia.Stem Cell Rev Rep. 2025 Jan;21(1):2-27. doi: 10.1007/s12015-024-10761-z. Epub 2024 Jul 8. Stem Cell Rev Rep. 2025. PMID: 38976142 Review.
-
The role of complement in the trafficking of hematopoietic stem/progenitor cells.Transfusion. 2012 Dec;52(12):2706-16. doi: 10.1111/j.1537-2995.2012.03636.x. Epub 2012 Apr 9. Transfusion. 2012. PMID: 22486360 Free PMC article. Review. No abstract available.
-
Signaling of the Complement Cleavage Product Anaphylatoxin C5a Through C5aR (CD88) Contributes to Pharmacological Hematopoietic Stem Cell Mobilization.Stem Cell Rev Rep. 2017 Dec;13(6):793-800. doi: 10.1007/s12015-017-9769-6. Stem Cell Rev Rep. 2017. PMID: 28918528 Free PMC article.
-
Complement or insult: the emerging link between complement cascade deficiencies and pathology of myeloid malignancies.J Leukoc Biol. 2024 Nov 4;116(5):966-984. doi: 10.1093/jleuko/qiae130. J Leukoc Biol. 2024. PMID: 38836653 Review.
References
-
- Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Curr Opin Organ Transplant. 2008;13:53–58. - PubMed
-
- Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp. 2009;57:1–9. - PubMed
-
- Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

