Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla
- PMID: 20153814
- PMCID: PMC2849863
- DOI: 10.1016/j.neuroscience.2010.02.012
Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla
Abstract
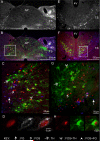
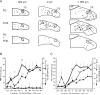
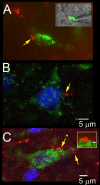
The nucleus tractus solitarius (nTS) of the brainstem receives sensory afferent inputs, processes that information, and sends projections to a variety of brain regions responsible for influencing autonomic and respiratory output. The nTS sends direct projections to the rostral ventrolateral medulla (RVLM), an area important for cardiorespiratory reflexes and homeostasis. Since the net reflex effect of nTS processing ultimately depends on the properties of output neurons, we determined the characteristics of these RVLM-projecting nTS neurons using electrophysiological and immunohistochemical techniques. RVLM-projecting nTS neurons were identified by retrograde tracers. Patch clamp analysis in the horizontal brainstem nTS slice demonstrated that RVLM-projecting nTS cells exhibit constant latency solitary tract evoked excitatory postsynaptic currents (EPSCs), suggesting they receive strong monosynaptic contacts from visceral afferents. Three distinct patterns of action potential firing, associated with different underlying potassium currents, were observed in RVLM-projecting cells. Following activation of the chemoreflex in conscious animals by 3 h of acute hypoxia, 11.2+/-1.9% of the RVLM-projecting nTS neurons were activated, as indicated by positive Fos-immunoreactivity. Very few RVLM-projecting nTS cells were catecholaminergic. Taken together, these data suggest that RVLM projecting nTS neurons receive strong monosynaptic inputs from sensory afferents and a subpopulation participates in the chemoreflex pathway.
(c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus.Am J Physiol Regul Integr Comp Physiol. 2012 May 15;302(10):R1219-32. doi: 10.1152/ajpregu.00028.2012. Epub 2012 Mar 7. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22403798 Free PMC article.
-
Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns.J Neurosci. 2006 Nov 15;26(46):11893-902. doi: 10.1523/JNEUROSCI.2044-06.2006. J Neurosci. 2006. PMID: 17108163 Free PMC article.
-
Membrane and synaptic properties of nucleus tractus solitarius neurons projecting to the caudal ventrolateral medulla.Auton Neurosci. 2007 Oct 30;136(1-2):69-81. doi: 10.1016/j.autneu.2007.04.006. Epub 2007 May 29. Auton Neurosci. 2007. PMID: 17537680
-
Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei.Clin Exp Pharmacol Physiol. 2005 May-Jun;32(5-6):450-6. doi: 10.1111/j.1440-1681.2005.04210.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15854157 Review.
-
Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc.Cell Mol Neurobiol. 2003 Oct;23(4-5):709-26. doi: 10.1023/a:1025096718559. Cell Mol Neurobiol. 2003. PMID: 14514026 Free PMC article. Review.
Cited by
-
Catecholaminergic neurons projecting to the paraventricular nucleus of the hypothalamus are essential for cardiorespiratory adjustments to hypoxia.Am J Physiol Regul Integr Comp Physiol. 2015 Oct;309(7):R721-31. doi: 10.1152/ajpregu.00540.2014. Epub 2015 Jul 8. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26157062 Free PMC article.
-
Nucleus Tractus Solitarius Neurons Activated by Hypercapnia and Hypoxia Lack Mu Opioid Receptor Expression.Front Mol Neurosci. 2022 Jul 11;15:932189. doi: 10.3389/fnmol.2022.932189. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35898697 Free PMC article.
-
5-hydroxytryptamine 2C receptors tonically augment synaptic currents in the nucleus tractus solitarii.J Neurophysiol. 2012 Oct;108(8):2292-305. doi: 10.1152/jn.00049.2012. Epub 2012 Aug 1. J Neurophysiol. 2012. PMID: 22855775 Free PMC article.
-
Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus.Am J Physiol Regul Integr Comp Physiol. 2012 May 15;302(10):R1219-32. doi: 10.1152/ajpregu.00028.2012. Epub 2012 Mar 7. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22403798 Free PMC article.
-
Circuit-Specific Control of Blood Pressure by PNMT-Expressing Nucleus Tractus Solitarii Neurons.Neurosci Bull. 2023 Aug;39(8):1193-1209. doi: 10.1007/s12264-022-01008-3. Epub 2023 Jan 2. Neurosci Bull. 2023. PMID: 36588135 Free PMC article.
References
-
- Aicher SA, Saravay RH, Cravo SL, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. - PubMed
-
- Andresen MC, Kunze DL. Nucleus tractus solitarius - gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
-
- Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol. 1997;77:2539–2547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

