Roles for the type III TGF-beta receptor in human cancer
- PMID: 20153821
- PMCID: PMC2875339
- DOI: 10.1016/j.cellsig.2010.01.016
Roles for the type III TGF-beta receptor in human cancer
Abstract
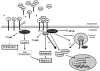
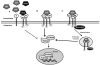
Transforming growth factor beta (TGF-beta) superfamily ligands have important roles in regulating cellular homeostasis, embryonic development, differentiation, proliferation, immune surveillance, angiogenesis, motility, and apoptosis in a cell type and context specific manner. TGF-beta superfamily signaling pathways also have diverse roles in human cancer, functioning to either suppress or promote cancer progression. The TGF-beta superfamily co-receptor, the type III TGF-beta receptor (TbetaRIII, also known as betaglycan) mediates TGF-beta superfamily ligand dependent as well as ligand independent signaling to both Smad and non-Smad signaling pathways. Loss of TbetaRIII expression during cancer progression and direct effects of TbetaRIII on regulating cell migration, invasion, proliferation, and angiogenesis support a role for TbetaRIII as a suppressor of cancer progression and/or as a metastasis suppressor. Defining the physiological function and mechanism of TbetaRIII action and alterations in TbetaRIII function during cancer progression should enable more effective targeting of TbetaRIII and TbetaRIII mediated functions for the diagnosis and treatment of human cancer.
Figures

Similar articles
-
Type III TGF-β receptor enhances colon cancer cell migration and anchorage-independent growth.Neoplasia. 2011 Aug;13(8):758-70. doi: 10.1593/neo.11528. Neoplasia. 2011. PMID: 21847367 Free PMC article.
-
Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression.Carcinogenesis. 2008 Feb;29(2):252-62. doi: 10.1093/carcin/bgm249. Epub 2007 Nov 13. Carcinogenesis. 2008. PMID: 17999987
-
The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling.Carcinogenesis. 2010 Feb;31(2):175-83. doi: 10.1093/carcin/bgp271. Epub 2009 Dec 2. Carcinogenesis. 2010. PMID: 19955393 Free PMC article.
-
TGF-β superfamily co-receptors in cancer.Dev Dyn. 2022 Jan;251(1):137-163. doi: 10.1002/dvdy.338. Epub 2021 Apr 9. Dev Dyn. 2022. PMID: 33797167 Free PMC article. Review.
-
The emerging role of TGF-beta superfamily coreceptors in cancer.Biochim Biophys Acta. 2009 Oct;1792(10):954-73. doi: 10.1016/j.bbadis.2009.07.003. Epub 2009 Jul 14. Biochim Biophys Acta. 2009. PMID: 19607914 Review.
Cited by
-
Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-β signaling.Neuron. 2013 May 8;78(3):456-68. doi: 10.1016/j.neuron.2013.03.004. Neuron. 2013. PMID: 23664613 Free PMC article.
-
Role of Betaglycan in TGF-β Signaling and Wound Healing in Human Endometriotic Epithelial Cells and in Endometriosis.Biology (Basel). 2022 Mar 26;11(4):513. doi: 10.3390/biology11040513. Biology (Basel). 2022. PMID: 35453712 Free PMC article.
-
Type III TGF-β receptor enhances colon cancer cell migration and anchorage-independent growth.Neoplasia. 2011 Aug;13(8):758-70. doi: 10.1593/neo.11528. Neoplasia. 2011. PMID: 21847367 Free PMC article.
-
Inhibin Is a Novel Paracrine Factor for Tumor Angiogenesis and Metastasis.Cancer Res. 2018 Jun 1;78(11):2978-2989. doi: 10.1158/0008-5472.CAN-17-2316. Epub 2018 Mar 13. Cancer Res. 2018. PMID: 29535220 Free PMC article.
-
Betaglycan (TβRIII) is expressed in the thymus and regulates T cell development by protecting thymocytes from apoptosis.PLoS One. 2012;7(8):e44217. doi: 10.1371/journal.pone.0044217. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952931 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

