ISG15 and immune diseases
- PMID: 20153823
- PMCID: PMC7127291
- DOI: 10.1016/j.bbadis.2010.02.006
ISG15 and immune diseases
Abstract
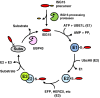
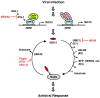
ISG15, the product of interferon (IFN)-stimulated gene 15, is the first identified ubiquitin-like protein, consisting of two ubiquitin-like domains. ISG15 is synthesized as a precursor in certain mammals and, therefore, needs to be processed to expose the C-terminal glycine residue before conjugation to target proteins. A set of three-step cascade enzymes, an E1 enzyme (UBE1L), an E2 enzyme (UbcH8), and one of several E3 ligases (e.g., EFP and HERC5), catalyzes ISG15 conjugation (ISGylation) of a specific protein. These enzymes are unique among the cascade enzymes for ubiquitin and other ubiquitin-like proteins in that all of them are induced by type I IFNs or other stimuli, such as exposure to viruses and lipopolysaccharide. Mass spectrometric analysis has led to the identification of several hundreds of candidate proteins that can be conjugated by ISG15. Some of them are type I IFN-induced proteins, such as PKR and RIG-I, and some are the key regulators that are involved in IFN signaling, such as JAK1 and STAT1, implicating the role of ISG15 and its conjugates in type I IFN-mediated innate immune responses. However, relatively little is known about the functional significance of ISG15 induction due to the lack of information on the consequences of its conjugation to target proteins. Here, we describe the recent progress made in exploring the biological function of ISG15 and its reversible modification of target proteins and thus in their implication in immune diseases.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells.J Biol Chem. 2006 Feb 17;281(7):4334-8. doi: 10.1074/jbc.M512830200. Epub 2005 Dec 28. J Biol Chem. 2006. PMID: 16407192
-
The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein.Proc Natl Acad Sci U S A. 2004 May 18;101(20):7578-82. doi: 10.1073/pnas.0402528101. Epub 2004 May 6. Proc Natl Acad Sci U S A. 2004. PMID: 15131269 Free PMC article.
-
The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase.J Biol Chem. 2006 Feb 17;281(7):3989-94. doi: 10.1074/jbc.M510787200. Epub 2005 Dec 13. J Biol Chem. 2006. PMID: 16352599
-
[Advances in ubiquitin-like protein ISG15-mediated anti-viral response].Bing Du Xue Bao. 2012 Jan;28(1):78-83. Bing Du Xue Bao. 2012. PMID: 22416355 Review. Chinese.
-
Coronaviral PLpro proteases and the immunomodulatory roles of conjugated versus free Interferon Stimulated Gene product-15 (ISG15).Semin Cell Dev Biol. 2022 Dec;132:16-26. doi: 10.1016/j.semcdb.2022.06.005. Epub 2022 Jun 25. Semin Cell Dev Biol. 2022. PMID: 35764457 Free PMC article. Review.
Cited by
-
Cuprizone and EAE mouse frontal cortex proteomics revealed proteins altered in multiple sclerosis.Sci Rep. 2021 Mar 30;11(1):7174. doi: 10.1038/s41598-021-86191-5. Sci Rep. 2021. PMID: 33785790 Free PMC article.
-
Ubiquitin-fold modifier 1 acts as a positive regulator of breast cancer.Front Endocrinol (Lausanne). 2015 Mar 20;6:36. doi: 10.3389/fendo.2015.00036. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25852645 Free PMC article. Review.
-
Antiviral Properties of ISG15.Viruses. 2010 Oct;2(10):2154-2168. doi: 10.3390/v2102154. Epub 2010 Sep 28. Viruses. 2010. PMID: 21994614 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 1 Interacts with a Member of the Interferon-Stimulated Gene 15 Pathway.J Virol. 2015 Nov;89(22):11572-83. doi: 10.1128/JVI.01482-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355087 Free PMC article.
-
Weighing up the possibilities: Controlling translation by ubiquitylation and sumoylation.Translation (Austin). 2014 Oct 30;2(2):e959366. doi: 10.4161/2169074X.2014.959366. eCollection 2014 Sep 1. Translation (Austin). 2014. PMID: 26779408 Free PMC article. Review.
References
-
- Isaacs A., Lindenmann J. Virus interference: I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957;147:258–267. - PubMed
-
- Darnell J.E., Jr., Kerr I.M., Stark G.R. Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. - PubMed
-
- Heim M.H., Kerr I.M., Stark G.R., Darnell J.E., Jr. Contribution of STAT SH2 groups to specific interferon signaling by the Jak–STAT pathway. Science. 1995;267:1347–1349. - PubMed
-
- Shuai K., Stark G.R., Kerr I.M., Darnell J.E., Jr. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993;261:1744–1746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

