Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial
- PMID: 20153888
- PMCID: PMC2877592
- DOI: 10.1016/S0140-6736(09)62038-9
Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial
Abstract
Background: Most people infected with HIV-1 are dually infected with herpes simplex virus type 2. Daily suppression of this herpes virus reduces plasma HIV-1 concentrations, but whether it delays HIV-1 disease progression is unknown. We investigated the effect of acyclovir on HIV-1 progression.
Methods: In a trial with 14 sites in southern Africa and east Africa, 3381 heterosexual people who were dually infected with herpes simplex virus type 2 and HIV-1 were randomly assigned in a 1:1 ratio to acyclovir 400 mg orally twice daily or placebo, and were followed up for up to 24 months. Eligible participants had CD4 cell counts of 250 cells per mL or higher and were not taking antiretroviral therapy. We used block randomisation, and patients and investigators were masked to treatment allocation. Effect of acyclovir on HIV-1 disease progression was defined by a primary composite endpoint of first occurrence of CD4 cell counts of fewer than 200 cells per microL, antiretroviral therapy initiation, or non-trauma related death. As an exploratory analysis, we assessed the endpoint of CD4 falling to <350 cells per microL. Analysis was by intention to treat. The trial is registered with ClinicalTrials.gov, number NCT00194519.
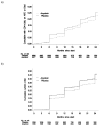
Findings: At enrollment, the median CD4 cell count was 462 cells per microL and median HIV-1 plasma RNA was 4.1 log(10) copies per microL. Acyclovir reduced risk of HIV-1 disease progression by 16%; 284 participants assigned acyclovir versus 324 assigned placebo reached the primary endpoint (hazard ratio [HR] 0.84, 95% CI 0.71-0.98, p=0.03). In those with CD4 counts >or=350 cells per microL, aciclovir delayed risk of CD4 cell counts falling to <350 cells per microL by 19% (0.81, 0.71-0.93, p=0.002)
Interpretation: The role of suppression of herpes simplex virus type 2 in reduction of HIV-1 disease progression before initiation of antiretroviral therapy warrants consideration.
Funding: Bill & Melinda Gates Foundation.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Treating HIV infection with drugs for HSV-2 infection?Lancet. 2010 Mar 6;375(9717):782-4. doi: 10.1016/S0140-6736(10)60097-9. Epub 2010 Feb 12. Lancet. 2010. PMID: 20153889 No abstract available.
Similar articles
-
Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial.Lancet Infect Dis. 2012 Jun;12(6):441-8. doi: 10.1016/S1473-3099(12)70037-3. Epub 2012 Mar 19. Lancet Infect Dis. 2012. PMID: 22433279 Free PMC article. Clinical Trial.
-
Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2.N Engl J Med. 2010 Feb 4;362(5):427-39. doi: 10.1056/NEJMoa0904849. Epub 2010 Jan 20. N Engl J Med. 2010. PMID: 20089951 Free PMC article. Clinical Trial.
-
Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus.N Engl J Med. 2007 Feb 22;356(8):790-9. doi: 10.1056/NEJMoa062607. N Engl J Med. 2007. PMID: 17314338 Clinical Trial.
-
Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections.Drugs. 2000 Apr;59(4):839-63. doi: 10.2165/00003495-200059040-00013. Drugs. 2000. PMID: 10804039 Review.
-
Recent developments in the management of herpes simplex virus infection in HIV-infected persons.Clin Infect Dis. 2004 Nov 1;39 Suppl 5:S237-9. doi: 10.1086/422360. Clin Infect Dis. 2004. PMID: 15494894 Review. No abstract available.
Cited by
-
Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study.PLoS Med. 2011 Nov;8(11):e1001123. doi: 10.1371/journal.pmed.1001123. Epub 2011 Nov 15. PLoS Med. 2011. PMID: 22110407 Free PMC article.
-
Use of injectable hormonal contraception and HSV-2 acquisition in a cohort of female sex workers in Vancouver, Canada.Sex Transm Infect. 2017 Jun;93(4):284-289. doi: 10.1136/sextrans-2016-052838. Epub 2016 Nov 7. Sex Transm Infect. 2017. PMID: 27821613 Free PMC article.
-
A prospective study of the effect of pregnancy on CD4 counts and plasma HIV-1 RNA concentrations of antiretroviral-naive HIV-1-infected women.J Acquir Immune Defic Syndr. 2014 Feb 1;65(2):231-6. doi: 10.1097/QAI.0000000000000013. J Acquir Immune Defic Syndr. 2014. PMID: 24442226 Free PMC article. Clinical Trial.
-
High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial.J Infect Dis. 2011 Dec 15;204(12):1912-7. doi: 10.1093/infdis/jir649. Epub 2011 Oct 12. J Infect Dis. 2011. PMID: 21998479 Free PMC article. Clinical Trial.
-
Development and application of modern agricultural biotechnology in Botswana: the potentials, opportunities and challenges.GM Crops Food. 2014 Jul 3;5(3):183-94. doi: 10.4161/21645698.2014.945887. GM Crops Food. 2014. PMID: 25437237 Free PMC article.
References
-
- UN AIDS/World Health Organization. Report on the global AIDS epidemic. Geneva: UNAIDS/World Health Organization; 2008. Dec,
-
- Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004 Apr;11( Suppl 1):24A–35A. - PubMed
-
- Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis. 2004 Aug 15;190(4):693–6. - PubMed
-
- Baeten JM, McClelland RS, Corey L, Overbaugh J, Lavreys L, Richardson BA, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004 Apr 15;189(8):1466–71. - PubMed
-
- Mbopi-Keou FX, Legoff J, Gresenguet G, Si-Mohamed A, Matta M, Mayaud P, et al. Genital shedding of herpes simplex virus-2 DNA and HIV-1 RNA and proviral DNA in HIV-1- and herpes simplex virus-2-coinfected African women. J Acquir Immune Defic Syndr. 2003 Jun 1;33(2):121–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

