A pharmacogenetic model of naltrexone-induced attenuation of alcohol consumption in rhesus monkeys
- PMID: 20153935
- PMCID: PMC2875311
- DOI: 10.1016/j.drugalcdep.2010.01.005
A pharmacogenetic model of naltrexone-induced attenuation of alcohol consumption in rhesus monkeys
Abstract
Background: Variation at the human mu-opioid receptor has been associated with alcohol abuse. The A118G (N40D) polymorphism in humans is functionally mimicked by the C77G (P26R) polymorphism in rhesus monkeys; both show similar in vitro influences on ligand binding and in vivo correlations with physiological measures as well as behavioral measures including predilection towards alcohol consumption. Naltrexone, an antagonist at the receptor, has been used to treat alcoholism in humans and has been reported to show differences in effectiveness depending on genotype.
Methods: Here we describe a study in which we a priori selected rhesus monkeys based on genotype at the OPRM1 C77G single nucleotide polymorphism, trained them to self-administer alcohol, and assessed naltrexone responsiveness.
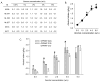
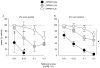
Results: Alcohol intake in rhesus monkeys varied with genotype across a range of alcohol concentrations (0.5-4%, w/v) such that animals with the G/G genotype drank consistently more alcohol than those animals with the C/C genotype. Additionally, naltrexone attenuated alcohol drinking in a dose- and genotype-dependent manner. Animals harboring the G/G genotype were more sensitive to the effects of naltrexone and showed greater reductions in alcohol consumption at lower naltrexone doses compared to animals with a C/G or C/C genotype.
Conclusions: This preliminary study demonstrates a pharmacogenomic response to naltrexone in rhesus monkeys that parallels that seen in humans. This finding provides a basis for developing a pharmacogenetic animal model for naltrexone effect that can expand further our understanding of the causes and treatments of alcohol use disorders.
Copyright (c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Suppression of alcohol preference by naltrexone in the rhesus macaque: a critical role of genetic variation at the micro-opioid receptor gene locus.Biol Psychiatry. 2010 Jan 1;67(1):78-80. doi: 10.1016/j.biopsych.2009.07.026. Biol Psychiatry. 2010. PMID: 19748082 Free PMC article.
-
Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study.Arch Gen Psychiatry. 2007 Sep;64(9):1069-77. doi: 10.1001/archpsyc.64.9.1069. Arch Gen Psychiatry. 2007. PMID: 17768272 Clinical Trial.
-
A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice.Biol Psychiatry. 2015 May 15;77(10):850-8. doi: 10.1016/j.biopsych.2014.08.021. Epub 2014 Sep 8. Biol Psychiatry. 2015. PMID: 25442002
-
Systematic review and meta-analysis of the moderating effect of rs1799971 in OPRM1, the mu-opioid receptor gene, on response to naltrexone treatment of alcohol use disorder.Addiction. 2020 Aug;115(8):1426-1437. doi: 10.1111/add.14975. Epub 2020 Feb 11. Addiction. 2020. PMID: 31961981 Free PMC article.
-
Influence of the OPRM1 A118G polymorphism on alcohol-induced euphoria, risk for alcoholism and the clinical efficacy of naltrexone.Pharmacogenomics. 2012 Jul;13(10):1161-72. doi: 10.2217/pgs.12.99. Pharmacogenomics. 2012. PMID: 22909206 Review.
Cited by
-
Opioid pharmacogenetics of alcohol addiction.Cold Spring Harb Perspect Med. 2013 Jul 1;3(7):a012203. doi: 10.1101/cshperspect.a012203. Cold Spring Harb Perspect Med. 2013. PMID: 23729643 Free PMC article. Review.
-
Whole-genome characterization in pedigreed non-human primates using genotyping-by-sequencing (GBS) and imputation.BMC Genomics. 2016 Aug 24;17(1):676. doi: 10.1186/s12864-016-2966-x. BMC Genomics. 2016. PMID: 27558348 Free PMC article.
-
Increased ethanol drinking in "humanized" mice expressing the mu opioid receptor A118G polymorphism are mediated through sex-specific mechanisms.Brain Res Bull. 2018 Apr;138:12-19. doi: 10.1016/j.brainresbull.2017.07.017. Epub 2017 Aug 2. Brain Res Bull. 2018. PMID: 28780411 Free PMC article.
-
Pharmacogenetic approaches to the treatment of alcohol addiction.Nat Rev Neurosci. 2011 Oct 20;12(11):670-84. doi: 10.1038/nrn3110. Nat Rev Neurosci. 2011. PMID: 22011682 Free PMC article. Review.
-
Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes.Alcohol Clin Exp Res. 2012 Nov;36(11):2000-7. doi: 10.1111/j.1530-0277.2012.01807.x. Epub 2012 May 2. Alcohol Clin Exp Res. 2012. PMID: 22551036 Free PMC article. Clinical Trial.
References
-
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. - PMC - PubMed
-
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64:369–376. - PubMed
-
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AA016828/AA/NIAAA NIH HHS/United States
- R01 AA016828/AA/NIAAA NIH HHS/United States
- K02 DA025697/DA/NIDA NIH HHS/United States
- MH077995/MH/NIMH NIH HHS/United States
- R01 AA016179/AA/NIAAA NIH HHS/United States
- AA016194/AA/NIAAA NIH HHS/United States
- AA016179/AA/NIAAA NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- F32 MH082507/MH/NIMH NIH HHS/United States
- R21 AA016194/AA/NIAAA NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- R21 MH077995/MH/NIMH NIH HHS/United States
- DA025697/DA/NIDA NIH HHS/United States
- MH082507/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

