Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis
- PMID: 20153989
- PMCID: PMC2836402
- DOI: 10.1016/j.dld.2010.01.008
Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis
Abstract
Bile duct epithelial cells (i.e., cholangiocytes), which line the intrahepatic biliary epithelium, are the target cells in a number of human cholestatic liver diseases (termed cholangiopathies). Cholangiocyte proliferation and death is present in virtually all human cholangiopathies. A number of recent studies have provided insights into the key mechanisms that regulate the proliferation and function of cholangiocytes during the pathogenesis of cholestatic liver diseases. In our review, we have summarised the most important of these recent studies over the past 3 years with a focus on those performed in the animal model of extrahepatic bile duct ligation. In the first part of the review, we provide relevant background on the biliary ductal system. We then proceed with a general discussion of the factors regulating biliary proliferation performed in the cholestatic animal model of bile duct ligation. Further characterisation of the factors that regulate cholangiocyte proliferation and function will help in elucidating the mechanisms regulating the pathogenesis of biliary tract diseases in humans and in devising new treatment approaches for these devastating diseases.
Copyright (c) 2010 Editrice Gastroenterologica Italiana S.r.l. All rights reserved.
Conflict of interest statement
Figures

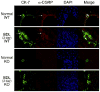
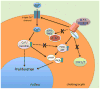

Similar articles
-
Regulators of Cholangiocyte Proliferation.Gene Expr. 2017 Feb 10;17(2):155-171. doi: 10.3727/105221616X692568. Epub 2016 Jul 12. Gene Expr. 2017. PMID: 27412505 Free PMC article. Review.
-
Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation.Hepatology. 2010 Jul;52(1):204-14. doi: 10.1002/hep.23657. Hepatology. 2010. PMID: 20578263 Free PMC article.
-
Mechanisms of cholangiocyte responses to injury.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1262-1269. doi: 10.1016/j.bbadis.2017.06.017. Epub 2017 Jun 23. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28648950 Free PMC article. Review.
-
Cholangiocyte pathobiology.Nat Rev Gastroenterol Hepatol. 2019 May;16(5):269-281. doi: 10.1038/s41575-019-0125-y. Nat Rev Gastroenterol Hepatol. 2019. PMID: 30850822 Free PMC article. Review.
-
Bile duct ligation: step-by-step to cholangiocyte inflammatory tumorigenesis.Eur J Gastroenterol Hepatol. 2010 Jun;22(6):651-61. doi: 10.1097/MEG.0b013e32832e0a2f. Eur J Gastroenterol Hepatol. 2010. PMID: 19641467 Review.
Cited by
-
α7-nAChR Knockout Mice Decreases Biliary Hyperplasia and Liver Fibrosis in Cholestatic Bile Duct-Ligated Mice.Gene Expr. 2018 Aug 22;18(3):197-207. doi: 10.3727/105221618X15216453076707. Epub 2018 Mar 26. Gene Expr. 2018. PMID: 29580318 Free PMC article.
-
Immunohistochemical study for the origin of ductular reaction in chronic liver disease.Int J Clin Exp Pathol. 2014 Jun 15;7(7):4076-85. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25120786 Free PMC article.
-
Role of follicle-stimulating hormone on biliary cyst growth in autosomal dominant polycystic kidney disease.Liver Int. 2013 Jul;33(6):914-25. doi: 10.1111/liv.12177. Epub 2013 Apr 25. Liver Int. 2013. PMID: 23617956 Free PMC article.
-
The emerging role of mast cells in liver disease.Am J Physiol Gastrointest Liver Physiol. 2017 Aug 1;313(2):G89-G101. doi: 10.1152/ajpgi.00333.2016. Epub 2017 May 4. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28473331 Free PMC article. Review.
-
CCN1 induces hepatic ductular reaction through integrin αvβ₅-mediated activation of NF-κB.J Clin Invest. 2015 May;125(5):1886-900. doi: 10.1172/JCI79327. Epub 2015 Mar 30. J Clin Invest. 2015. PMID: 25822023 Free PMC article.
References
-
- Alpini G, Prall RT, LaRussoF NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA, editors. The liver; biology & pathobiology. 4th. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 421–35.
-
- Kanno N, LeSage G, Glaser S, et al. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–61. - PubMed
-
- Sasaki H, Schaffner F, Popper H. Bile ductules in cholestasis: morphologic evidence for secretion and absorption in man. Lab Invest. 1967;16:84–95. - PubMed
-
- Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–66. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

