Retracted: Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors
- PMID: 20154087
- PMCID: PMC2857028
- DOI: 10.1074/jbc.M109.090209
Retracted: Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors
Retraction in
-
Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors.J Biol Chem. 2016 Aug 5;291(32):16920. doi: 10.1074/jbc.A109.090209. J Biol Chem. 2016. PMID: 27496961 Free PMC article. No abstract available.
Abstract
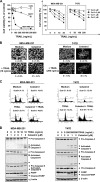
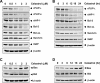
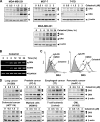
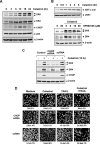
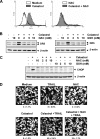
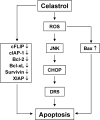
Whether celastrol, a triterpene from traditional Chinese medicine, can modulate the anticancer effects of TRAIL, the cytokine that is currently in clinical trial, was investigated. As indicated by assays that measure plasma membrane integrity, phosphatidylserine exposure, mitochondrial activity, and activation of caspase-8, caspase-9, and caspase-3, celastrol potentiated the TRAIL-induced apoptosis in human breast cancer cells, and converted TRAIL-resistant cells to TRAIL-sensitive cells. When examined for its mechanism, we found that the triterpene down-regulated the expression of cell survival proteins including cFLIP, IAP-1, Bcl-2, Bcl-xL, survivin, and XIAP and up-regulated Bax expression. In addition, we found that celastrol induced the cell surface expression of both the TRAIL receptors DR4 and DR5. This increase in receptors was noted in a wide variety of cancer cells including breast, lung, colorectal, prostate, esophageal, and pancreatic cancer cells, and myeloid and leukemia cells. Gene silencing of the death receptor abolished the effect of celastrol on TRAIL-induced apoptosis. Induction of the death receptor by the triterpenoid was found to be p53-independent but required the induction of CAAT/enhancer-binding protein homologous protein (CHOP), inasmuch as gene silencing of CHOP abolished the induction of DR5 expression by celastrol and associated enhancement of TRAIL-induced apoptosis. We found that celastrol also induced reactive oxygen species (ROS) generation, and ROS sequestration inhibited celastrol-induced expression of CHOP and DR5, and consequent sensitization to TRAIL. Overall, our results demonstrate that celastrol can potentiate the apoptotic effects of TRAIL through down-regulation of cell survival proteins and up-regulation of death receptors via the ROS-mediated up-regulation of CHOP pathway.
Figures

References
-
- Wiley S. R., Schooley K., Smolak P. J., Din W. S., Huang C. P., Nicholl J. K., Sutherland G. R., Smith T. D., Rauch C., Smith C. A. (1995) Immunity 3, 673–682 - PubMed
-
- Pitti R. M., Marsters S. A., Ruppert S., Donahue C. J., Moore A., Ashkenazi A. (1996) J. Biol. Chem. 271, 12687–12690 - PubMed
-
- Bhardwaj A., Aggarwal B. B. (2003) J. Clin. Immunol. 23, 317–332 - PubMed
-
- Rowinsky E. K. (2005) J. Clin. Oncol. 23, 9394–9407 - PubMed
-
- Wagner K. W., Punnoose E. A., Januario T., Lawrence D. A., Pitti R. M., Lancaster K., Lee D., von Goetz M., Yee S. F., Totpal K., Huw L., Katta V., Cavet G., Hymowitz S. G., Amler L., Ashkenazi A. (2007) Nat. Med. 13, 1070–1077 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

