Phytochrome regulation of branching in Arabidopsis
- PMID: 20154098
- PMCID: PMC2850038
- DOI: 10.1104/pp.109.148833
Phytochrome regulation of branching in Arabidopsis
Abstract
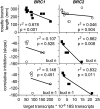
The red light:far-red light ratio perceived by phytochromes controls plastic traits of plant architecture, including branching. Despite the significance of branching for plant fitness and productivity, there is little quantitative and mechanistic information concerning phytochrome control of branching responses in Arabidopsis (Arabidopsis thaliana). Here, we show that in Arabidopsis, the negative effects of the phytochrome B mutation and of low red light:far-red light ratio on branching were largely due to reduced bud outgrowth capacity and an increased degree of correlative inhibition acting on the buds rather than due to a reduced number of leaves and buds available for branching. Phytochrome effects on the degree of correlative inhibition required functional BRANCHED1 (BRC1), BRC2, AXR1, MORE AXILLARY GROWTH2 (MAX2), and MAX4. The analysis of gene expression in selected buds indicated that BRC1 and BRC2 are part of different gene networks. The BRC1 network is linked to the growth capacity of specific buds, while the BRC2 network is associated with coordination of growth among branches. We conclude that the branching integrators BRC1 and BRC2 are necessary for responses to phytochrome, but they contribute differentially to these responses, likely acting through divergent pathways.
Figures








References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Ballaré CL. (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97–102 - PubMed
-
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 - PubMed
-
- Boe A, Beck DL. (2008) Yield components of biomass in switchgrass. Crop Sci 48: 1306–1311
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

