Requirements for construction of a functional hybrid complex of photosystem I and [NiFe]-hydrogenase
- PMID: 20154103
- PMCID: PMC2849213
- DOI: 10.1128/AEM.02700-09
Requirements for construction of a functional hybrid complex of photosystem I and [NiFe]-hydrogenase
Abstract
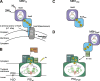
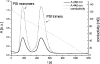
The development of cellular systems in which the enzyme hydrogenase is efficiently coupled to the oxygenic photosynthesis apparatus represents an attractive avenue to produce H(2) sustainably from light and water. Here we describe the molecular design of the individual components required for the direct coupling of the O(2)-tolerant membrane-bound hydrogenase (MBH) from Ralstonia eutropha H16 to the acceptor site of photosystem I (PS I) from Synechocystis sp. PCC 6803. By genetic engineering, the peripheral subunit PsaE of PS I was fused to the MBH, and the resulting hybrid protein was purified from R. eutropha to apparent homogeneity via two independent affinity chromatographical steps. The catalytically active MBH-PsaE (MBH(PsaE)) hybrid protein could be isolated only from the cytoplasmic fraction. This was surprising, since the MBH is a substrate of the twin-arginine translocation system and was expected to reside in the periplasm. We conclude that the attachment of the additional PsaE domain to the small, electron-transferring subunit of the MBH completely abolished the export competence of the protein. Activity measurements revealed that the H(2) production capacity of the purified MBH(PsaE) fusion protein was very similar to that of wild-type MBH. In order to analyze the specific interaction of MBH(PsaE) with PS I, His-tagged PS I lacking the PsaE subunit was purified via Ni-nitrilotriacetic acid affinity and subsequent hydrophobic interaction chromatography. Formation of PS I-hydrogenase supercomplexes was demonstrated by blue native gel electrophoresis. The results indicate a vital prerequisite for the quantitative analysis of the MBH(PsaE)-PS I complex formation and its light-driven H(2) production capacity by means of spectroelectrochemistry.
Figures
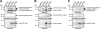
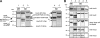



Similar articles
-
Light-driven hydrogen production by a hybrid complex of a [NiFe]-hydrogenase and the cyanobacterial photosystem I.Photochem Photobiol. 2006 May-Jun;82(3):676-82. doi: 10.1562/2006-01-16-RA-778. Photochem Photobiol. 2006. PMID: 16542111
-
Photoinduced hydrogen production by direct electron transfer from photosystem I cross-linked with cytochrome c3 to [NiFe]-hydrogenase.Photochem Photobiol. 2006 Nov-Dec;82(6):1677-85. doi: 10.1562/2006-05-07-RA-893. Photochem Photobiol. 2006. PMID: 16836469
-
A trimeric supercomplex of the oxygen-tolerant membrane-bound [NiFe]-hydrogenase from Ralstonia eutropha H16.Biochemistry. 2011 Dec 20;50(50):10836-43. doi: 10.1021/bi201594m. Epub 2011 Nov 29. Biochemistry. 2011. PMID: 22097922
-
[NiFe]-hydrogenases of Ralstonia eutropha H16: modular enzymes for oxygen-tolerant biological hydrogen oxidation.J Mol Microbiol Biotechnol. 2005;10(2-4):181-96. doi: 10.1159/000091564. J Mol Microbiol Biotechnol. 2005. PMID: 16645314 Review.
-
H2 conversion in the presence of O2 as performed by the membrane-bound [NiFe]-hydrogenase of Ralstonia eutropha.Chemphyschem. 2010 Apr 26;11(6):1107-19. doi: 10.1002/cphc.200901002. Chemphyschem. 2010. PMID: 20186906 Review.
Cited by
-
Autotrophic production of stable-isotope-labeled arginine in Ralstonia eutropha strain H16.Appl Environ Microbiol. 2012 Nov;78(22):7884-90. doi: 10.1128/AEM.01972-12. Epub 2012 Aug 31. Appl Environ Microbiol. 2012. PMID: 22941075 Free PMC article.
-
Development of a transferable bimolecular fluorescence complementation system for the investigation of interactions between poly(3-hydroxybutyrate) granule-associated proteins in Gram-negative bacteria.Appl Environ Microbiol. 2013 May;79(9):2989-99. doi: 10.1128/AEM.03965-12. Epub 2013 Feb 22. Appl Environ Microbiol. 2013. PMID: 23435892 Free PMC article.
-
Implementation of a high cell density fed-batch for heterologous production of active [NiFe]-hydrogenase in Escherichia coli bioreactor cultivations.Microb Cell Fact. 2022 Sep 19;21(1):193. doi: 10.1186/s12934-022-01919-w. Microb Cell Fact. 2022. PMID: 36123684 Free PMC article.
-
Exploring Structure and Function of Redox Intermediates in [NiFe]-Hydrogenases by an Advanced Experimental Approach for Solvated, Lyophilized and Crystallized Metalloenzymes.Angew Chem Int Ed Engl. 2021 Jul 12;60(29):15854-15862. doi: 10.1002/anie.202100451. Epub 2021 May 5. Angew Chem Int Ed Engl. 2021. PMID: 33783938 Free PMC article.
-
Synthetic biology toolkit for engineering Cupriviadus necator H16 as a platform for CO2 valorization.Biotechnol Biofuels. 2021 Nov 4;14(1):212. doi: 10.1186/s13068-021-02063-0. Biotechnol Biofuels. 2021. PMID: 34736496 Free PMC article. Review.
References
-
- Asada, Y., and J. Miyake. 1999. Photobiological hydrogen production. J. Biosci. Bioeng. 88:1-6. - PubMed
-
- Badura, A., B. Esper, K. Ataka, C. Grunwald, C. Woll, J. Kuhlmann, J. Heberle, and M. Rögner. 2006. Light-driven water splitting for (bio-)hydrogen production: photosystem 2 as the central part of a bioelectrochemical device. Photochem. Photobiol. 82:1385-1390. - PubMed
-
- Benemann, J. R. 2000. Hydrogen production by microalgae. J. Appl. Phycol. 12:291-300.
-
- Bernhard, M., B. Benelli, A. Hochkoeppler, D. Zannoni, and B. Friedrich. 1997. Functional and structural role of the cytochrome b subunit of the membrane-bound hydrogenase complex of Alcaligenes eutrophus H16. Eur. J. Biochem. 248:179-186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

