Organization of the electron transfer chain to oxygen in the obligate human pathogen Neisseria gonorrhoeae: roles for cytochromes c4 and c5, but not cytochrome c2, in oxygen reduction
- PMID: 20154126
- PMCID: PMC2863483
- DOI: 10.1128/JB.00002-10
Organization of the electron transfer chain to oxygen in the obligate human pathogen Neisseria gonorrhoeae: roles for cytochromes c4 and c5, but not cytochrome c2, in oxygen reduction
Abstract
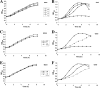
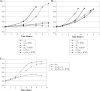
Although Neisseria gonorrhoeae is a prolific source of eight c-type cytochromes, little is known about how its electron transfer pathways to oxygen are organized. In this study, the roles in the respiratory chain to oxygen of cytochromes c(2), c(4), and c(5), encoded by the genes cccA, cycA, and cycB, respectively, have been investigated. Single mutations in genes for either cytochrome c(4) or c(5) resulted in an increased sensitivity to growth inhibition by excess oxygen and small decreases in the respiratory capacity of the parent, which were complemented by the chromosomal integration of an ectopic, isopropyl-beta-d-thiogalactopyranoside (IPTG)-inducible copy of the cycA or cycB gene. In contrast, a cccA mutant reduced oxygen slightly more rapidly than the parent, suggesting that cccA is expressed but cytochrome c(2) is not involved in electron transfer to cytochrome oxidase. The deletion of cccA increased the sensitivity of the cycB mutant to excess oxygen but decreased the sensitivity of the cycA mutant. Despite many attempts, a double mutant defective in both cytochromes c(4) and c(5) could not be isolated. However, a strain with the ectopically encoded, IPTG-inducible cycB gene with deletions in both cycA and cycB was constructed: the growth and survival of this strain were dependent upon the addition of IPTG, so gonococcal survival is dependent upon the synthesis of either cytochrome c(4) or c(5). These results define the gonococcal electron transfer chain to oxygen in which cytochromes c(4) and c(5), but not cytochrome c(2), provide alternative pathways for electron transfer from the cytochrome bc(1) complex to the terminal oxidase cytochrome cbb(3).
Figures

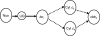
Similar articles
-
A critical role for the cccA gene product, cytochrome c2, in diverting electrons from aerobic respiration to denitrification in Neisseria gonorrhoeae.J Bacteriol. 2013 Jun;195(11):2518-29. doi: 10.1128/JB.02300-12. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543713 Free PMC article.
-
Roles of c-type cytochromes in respiration in Neisseria meningitidis.Microbiology (Reading). 2008 Sep;154(Pt 9):2857-2864. doi: 10.1099/mic.0.2008/020339-0. Microbiology (Reading). 2008. PMID: 18757819
-
Rhodobacter capsulatus CycH: a bipartite gene product with pleiotropic effects on the biogenesis of structurally different c-type cytochromes.J Bacteriol. 1996 Sep;178(17):5279-90. doi: 10.1128/jb.178.17.5279-5290.1996. J Bacteriol. 1996. PMID: 8752349 Free PMC article.
-
Soluble cytochrome c-554, CycA, is not essential for photosynthetic electron transfer in Chlorobium tepidum.FEBS Lett. 2006 Apr 17;580(9):2191-4. doi: 10.1016/j.febslet.2006.03.016. Epub 2006 Mar 15. FEBS Lett. 2006. PMID: 16579991
-
Structural and functional studies on the tetraheme cytochrome subunit and its electron donor proteins: the possible docking mechanisms during the electron transfer reaction.Photosynth Res. 2005;85(1):87-99. doi: 10.1007/s11120-004-2416-5. Photosynth Res. 2005. PMID: 15977061 Review.
Cited by
-
Expression of multiple cbb3 cytochrome c oxidase isoforms by combinations of multiple isosubunits in Pseudomonas aeruginosa.Proc Natl Acad Sci U S A. 2016 Nov 8;113(45):12815-12819. doi: 10.1073/pnas.1613308113. Epub 2016 Oct 24. Proc Natl Acad Sci U S A. 2016. PMID: 27791152 Free PMC article.
-
Cytochrome c4 is required for siderophore expression by Legionella pneumophila, whereas cytochromes c1 and c5 promote intracellular infection.Microbiology (Reading). 2011 Mar;157(Pt 3):868-878. doi: 10.1099/mic.0.046490-0. Epub 2010 Dec 22. Microbiology (Reading). 2011. PMID: 21178169 Free PMC article.
-
The structure of the diheme cytochrome c4 from Neisseria gonorrhoeae reveals multiple contributors to tuning reduction potentials.J Inorg Biochem. 2024 Apr;253:112496. doi: 10.1016/j.jinorgbio.2024.112496. Epub 2024 Jan 24. J Inorg Biochem. 2024. PMID: 38330683 Free PMC article.
-
The diheme cytochrome c(4) from Vibrio cholerae is a natural electron donor to the respiratory cbb(3) oxygen reductase.Biochemistry. 2010 Sep 7;49(35):7494-503. doi: 10.1021/bi1004574. Biochemistry. 2010. PMID: 20715760 Free PMC article.
-
High dissolved oxygen tension triggers outer membrane vesicle formation by Neisseria meningitidis.Microb Cell Fact. 2018 Oct 3;17(1):157. doi: 10.1186/s12934-018-1007-7. Microb Cell Fact. 2018. PMID: 30285743 Free PMC article.
References
-
- Deeudom, M., J. Rock, and J. Moir. 2006. Organization of the respiratory chain of Neisseria meningitidis. Biochem. Soc. Trans. 34:139-142. - PubMed
-
- de Gier, J. W. L., M. Schepper, W. N. M. Reijnders, S. J. van Dyke, D. J. Slotboom, A. Warne, M. Saraste, K. Krab, M. Finel, A. H. Stouthamer, R. J. M. van Spanning, and J. van der Oost. 1996. Structural and functional analysis of aa3-type and cbb3-type cytochrome c oxidases of Paracoccus denitrificans reveals significant differences in proton-pump design. Mol. Microbiol. 20:1247-1260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

