Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel
- PMID: 20154130
- PMCID: PMC2901697
- DOI: 10.1128/JB.01631-09
Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel
Abstract
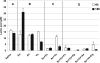
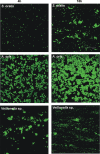
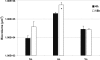
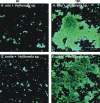
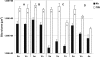
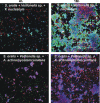
Human dental biofilm communities comprise several species, which can interact cooperatively or competitively. Bacterial interactions influence biofilm formation, metabolic changes, and physiological function of the community. Lactic acid, a common metabolite of oral bacteria, was measured in the flow cell effluent of one-, two- and three-species communities growing on saliva as the sole nutritional source. We investigated single-species and multispecies colonization by using known initial, early, middle, and late colonizers of enamel. Fluorescent-antibody staining and image analysis were used to quantify the biomass in saliva-fed flow cells. Of six species tested, only the initial colonizer Actinomyces oris exhibited significant growth. The initial colonizer Streptococcus oralis produced lactic acid but showed no significant growth. The early colonizer Veillonella sp. utilized lactic acid in two- and three-species biofilm communities. The biovolumes of all two-species biofilms increased when Veillonella sp. was present as one of the partners, indicating that this early colonizer promotes mutualistic community development. All three-species combinations exhibited enhanced growth except one, i.e., A. oris, Veillonella sp., and the middle colonizer Porphyromonas gingivalis, indicating specificity among three-species communities. Further specificity was seen when Fusobacterium nucleatum (a middle colonizer), Aggregatibacter actinomycetemcomitans (a late colonizer), and P. gingivalis did not grow with S. oralis in two-species biofilms, but inclusion of Veillonella sp. resulted in growth of all three-species combinations. We propose that commensal veillonellae use lactic acid for growth in saliva and that they communicate metabolically with initial, early, middle, and late colonizers to establish multispecies communities on enamel.
Figures

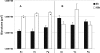
Similar articles
-
Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel.J Bacteriol. 2009 Nov;191(22):6804-11. doi: 10.1128/JB.01006-09. Epub 2009 Sep 11. J Bacteriol. 2009. PMID: 19749049 Free PMC article.
-
Aggregatibacter actinomycetemcomitans builds mutualistic biofilm communities with Fusobacterium nucleatum and Veillonella species in saliva.Infect Immun. 2009 Sep;77(9):3542-51. doi: 10.1128/IAI.00345-09. Epub 2009 Jun 29. Infect Immun. 2009. PMID: 19564387 Free PMC article.
-
Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source.Int J Oral Sci. 2011 Apr;3(2):49-54. doi: 10.4248/IJOS11025. Int J Oral Sci. 2011. PMID: 21485308 Free PMC article. Review.
-
Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota.J Periodontal Res. 2011 Apr;46(2):252-60. doi: 10.1111/j.1600-0765.2010.01341.x. Epub 2011 Jan 25. J Periodontal Res. 2011. PMID: 21261622
-
Saliva as the Sole Nutritional Source in the Development of Multispecies Communities in Dental Plaque.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0013-2014. Microbiol Spectr. 2015. PMID: 26185065 Review.
Cited by
-
Role of Oral Veillonella Species in Predicting Surgical Site Infections After Maxillofacial Trauma: A Prospective Observational Study.Cureus. 2024 Aug 5;16(8):e66158. doi: 10.7759/cureus.66158. eCollection 2024 Aug. Cureus. 2024. PMID: 39238733 Free PMC article.
-
Identification and characterization of a haem biosynthesis locus in Veillonella.Microbiology (Reading). 2016 Oct;162(10):1735-1743. doi: 10.1099/mic.0.000366. Epub 2016 Aug 26. Microbiology (Reading). 2016. PMID: 27566661 Free PMC article.
-
Oral multispecies biofilm development and the key role of cell-cell distance.Nat Rev Microbiol. 2010 Jul;8(7):471-80. doi: 10.1038/nrmicro2381. Nat Rev Microbiol. 2010. PMID: 20514044 Review.
-
Putative Microbial Population Shifts Attributable to Nasal Administration of Streptococcus salivarius 24SMBc and Streptococcus oralis 89a.Probiotics Antimicrob Proteins. 2019 Dec;11(4):1219-1226. doi: 10.1007/s12602-018-9488-6. Probiotics Antimicrob Proteins. 2019. PMID: 30535674 Free PMC article.
-
Genomic Diversity among Actinomyces naeslundii Strains and Closely Related Species.Microorganisms. 2023 Jan 19;11(2):254. doi: 10.3390/microorganisms11020254. Microorganisms. 2023. PMID: 36838222 Free PMC article.
References
-
- Bos, R., H. C. van der Mei, and H. J. Busscher. 1996. Co-adhesion of oral microbial pairs under flow in the presence of saliva and lactose. J. Dent. Res. 75:809-815. - PubMed
-
- Dawes, C., S. Watanabe, P. Biglow-Lecomte, and G. H. Dibdin. 1989. Estimation of the velocity of the salivary film at some different locations in the mouth. J. Dent. Res. 68:1479-1482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

