Role of FlgT in anchoring the flagellum of Vibrio cholerae
- PMID: 20154133
- PMCID: PMC2849446
- DOI: 10.1128/JB.01562-09
Role of FlgT in anchoring the flagellum of Vibrio cholerae
Abstract
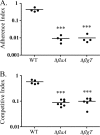
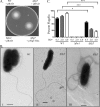
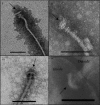
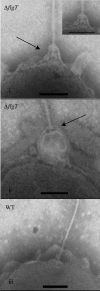
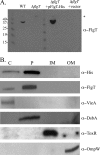
Flagellar motility has long been regarded as an important virulence factor. In Vibrio cholerae, the single polar flagellum is essential for motility as well as for proper attachment and colonization. In this study, we demonstrate that the novel flagellar protein FlgT is involved in anchoring the flagellum to the V. cholerae cell. A screen for novel colonization factors by use of TnphoA mutagenesis identified flgT. An in-frame deletion of flgT established that FlgT is required for attachment, colonization, and motility. Transmission electron microscopy revealed that while the flgT mutant is capable of assembling a phenotypically normal flagellum, the flgT population is mostly aflagellate compared to the wild-type population. Further analyses indicated that the flagellum of the flgT mutant is released into the culture supernatant from the cell upon completion of assembly. Additionally, hook basal body complexes appear to be released along with the filament. These results indicate that FlgT functions to stabilize the flagellar apparatus at the pole of the cell.
Figures


References
-
- Apel, D., and M. G. Surette. 2008. Bringing order to a complex molecular machine: the assembly of the bacterial flagella. Biochim. Biophys. Acta 1778:1851-1858. - PubMed
-
- Attmannspacher, U., B. E. Scharf, and R. M. Harshey. 2008. FliL is essential for swarming: motor rotation in absence of FliL fractures the flagellar rod in swarmer cells of Salmonella enterica. Mol. Microbiol. 68:328-341. - PubMed
-
- Attridge, S. R., and D. Rowley. 1983. The role of the flagellum in the adherence of Vibrio cholerae. J. Infect. Dis. 147:864-872. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

