The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation
- PMID: 20154145
- PMCID: PMC2849469
- DOI: 10.1128/MCB.01199-09
The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation
Abstract
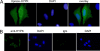
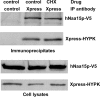
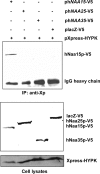
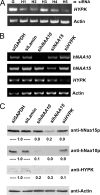
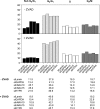
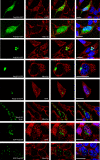
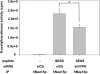
The human NatA protein N(alpha)-terminal-acetyltransferase complex is responsible for cotranslational N-terminal acetylation of proteins with Ser, Ala, Thr, Gly, and Val N termini. The NatA complex is composed of the catalytic subunit hNaa10p (hArd1) and the auxiliary subunit hNaa15p (hNat1/NATH). Using immunoprecipitation coupled with mass spectrometry, we identified endogenous HYPK, a Huntingtin (Htt)-interacting protein, as a novel stable interactor of NatA. HYPK has chaperone-like properties preventing Htt aggregation. HYPK, hNaa10p, and hNaa15p were associated with polysome fractions, indicating a function of HYPK associated with the NatA complex during protein translation. Knockdown of both hNAA10 and hNAA15 decreased HYPK protein levels, possibly indicating that NatA is required for the stability of HYPK. The biological importance of HYPK was evident from HYPK-knockdown HeLa cells displaying apoptosis and cell cycle arrest in the G(0)/G(1) phase. Knockdown of HYPK or hNAA10 resulted in increased aggregation of an Htt-enhanced green fluorescent protein (Htt-EGFP) fusion with expanded polyglutamine stretches, suggesting that both HYPK and NatA prevent Htt aggregation. Furthermore, we demonstrated that HYPK is required for N-terminal acetylation of the known in vivo NatA substrate protein PCNP. Taken together, the data indicate that the physical interaction between HYPK and NatA seems to be of functional importance both for Htt aggregation and for N-terminal acetylation.
Figures




Similar articles
-
A novel human NatA Nalpha-terminal acetyltransferase complex: hNaa16p-hNaa10p (hNat2-hArd1).BMC Biochem. 2009 May 29;10:15. doi: 10.1186/1471-2091-10-15. BMC Biochem. 2009. PMID: 19480662 Free PMC article.
-
Proteome-derived peptide libraries allow detailed analysis of the substrate specificities of N(alpha)-acetyltransferases and point to hNaa10p as the post-translational actin N(alpha)-acetyltransferase.Mol Cell Proteomics. 2011 May;10(5):M110.004580. doi: 10.1074/mcp.M110.004580. Epub 2011 Mar 7. Mol Cell Proteomics. 2011. PMID: 21383206 Free PMC article.
-
Structure of Human NatA and Its Regulation by the Huntingtin Interacting Protein HYPK.Structure. 2018 Jul 3;26(7):925-935.e8. doi: 10.1016/j.str.2018.04.003. Epub 2018 May 10. Structure. 2018. PMID: 29754825 Free PMC article.
-
The protein acetyltransferase ARD1: a novel cancer drug target?Curr Cancer Drug Targets. 2008 Nov;8(7):545-53. doi: 10.2174/156800908786241113. Curr Cancer Drug Targets. 2008. PMID: 18991565 Review.
-
N-acetyltransferase and inflammation: Bridging an unexplored niche.Gene. 2023 Dec 15;887:147730. doi: 10.1016/j.gene.2023.147730. Epub 2023 Aug 23. Gene. 2023. PMID: 37625560 Review.
Cited by
-
Hydroxylation of the Acetyltransferase NAA10 Trp38 Is Not an Enzyme-Switch in Human Cells.Int J Mol Sci. 2021 Oct 30;22(21):11805. doi: 10.3390/ijms222111805. Int J Mol Sci. 2021. PMID: 34769235 Free PMC article.
-
NAA10 p.(N101K) disrupts N-terminal acetyltransferase complex NatA and is associated with developmental delay and hemihypertrophy.Eur J Hum Genet. 2021 Feb;29(2):280-288. doi: 10.1038/s41431-020-00728-2. Epub 2020 Sep 24. Eur J Hum Genet. 2021. PMID: 32973342 Free PMC article.
-
Versatility of ARD1/NAA10-mediated protein lysine acetylation.Exp Mol Med. 2018 Jul 27;50(7):1-13. doi: 10.1038/s12276-018-0100-7. Exp Mol Med. 2018. PMID: 30054464 Free PMC article. Review.
-
Absence of N-terminal acetyltransferase diversification during evolution of eukaryotic organisms.Sci Rep. 2016 Feb 10;6:21304. doi: 10.1038/srep21304. Sci Rep. 2016. PMID: 26861501 Free PMC article.
-
Biochemical and cellular analysis of Ogden syndrome reveals downstream Nt-acetylation defects.Hum Mol Genet. 2015 Apr 1;24(7):1956-76. doi: 10.1093/hmg/ddu611. Epub 2014 Dec 8. Hum Mol Genet. 2015. PMID: 25489052 Free PMC article.
References
-
- Aiken, C. T., J. S. Steffan, C. M. Guerrero, H. Khashwji, T. Lukacsovich, D. Simmons, J. M. Purcell, K. Menhaji, Y. Z. Zhu, K. Green, F. Laferla, L. Huang, L. M. Thompson, and J. L. Marsh. 2009. Phosphorylation of threonine-3: implications for huntingtin aggregation and neurotoxicity. J. Biol. Chem. 284:29427-29436. - PMC - PubMed
-
- Ametzazurra, A., E. Larrea, M. P. Civeira, J. Prieto, and R. Aldabe. 2008. Implication of human N-alpha-acetyltransferase 5 in cellular proliferation and carcinogenesis. Oncogene 27:7296-7306. - PubMed
-
- Anderson, D. C., W. Li, D. G. Payan, and W. S. Noble. 2003. A new algorithm for the evaluation of shotgun peptide sequencing in proteomics: support vector machine classification of peptide MS/MS spectra and SEQUEST scores. J. Proteome Res. 2:137-146. - PubMed
-
- Arnesen, T., D. Anderson, J. Torsvik, H. B. Halseth, J. E. Varhaug, and J. R. Lillehaug. 2006. Cloning and characterization of hNAT5/hSAN: an evolutionarily conserved component of the NatA protein N-alpha-acetyltransferase complex. Gene 371:291-295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
