Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation
- PMID: 20154212
- PMCID: PMC2854427
- DOI: 10.1182/blood-2009-08-236729
Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation
Abstract
Runx1 is required for the emergence of hematopoietic stem cells (HSCs) from hemogenic endothelium during embryogenesis. However, its role in the generation and maintenance of HSCs during adult hematopoiesis remains uncertain. Here, we present analysis of a zebrafish mutant line carrying a truncation mutation, W84X, in runx1. The runx1(W84X/W84X) embryos showed blockage in the initiation of definitive hematopoiesis, but some embryos were able to recover from a larval "bloodless" phase and develop to fertile adults with multilineage hematopoiesis. Using cd41-green fluorescent protein transgenic zebrafish and lineage tracing, we demonstrated that the runx1(W84X/W84X) embryos developed cd41(+) HSCs in the aorta-gonad-mesonephros region, which later migrated to the kidney, the site of adult hematopoiesis. Overall, our data suggest that in zebrafish adult HSCs can be formed without an intact runx1.
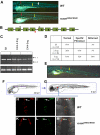
Figures

Similar articles
-
Redundant mechanisms driven independently by RUNX1 and GATA2 for hematopoietic development.Blood Adv. 2021 Dec 14;5(23):4949-4962. doi: 10.1182/bloodadvances.2020003969. Blood Adv. 2021. PMID: 34492681 Free PMC article.
-
CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis.Development. 2008 May;135(10):1853-62. doi: 10.1242/dev.015297. Epub 2008 Apr 16. Development. 2008. PMID: 18417622 Free PMC article.
-
CBFβ and RUNX1 are required at 2 different steps during the development of hematopoietic stem cells in zebrafish.Blood. 2014 Jul 3;124(1):70-8. doi: 10.1182/blood-2013-10-531988. Epub 2014 May 21. Blood. 2014. PMID: 24850758 Free PMC article.
-
Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo.Curr Top Dev Biol. 2003;53:139-58. doi: 10.1016/s0070-2153(03)53004-6. Curr Top Dev Biol. 2003. PMID: 12510667 Review.
-
Hematopoiesis.Development. 2013 Jun;140(12):2463-7. doi: 10.1242/dev.083147. Development. 2013. PMID: 23715539 Free PMC article. Review.
Cited by
-
The zebrafish: A fintastic model for hematopoietic development and disease.Wiley Interdiscip Rev Dev Biol. 2018 May;7(3):e312. doi: 10.1002/wdev.312. Epub 2018 Feb 13. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29436122 Free PMC article. Review.
-
CHD7 and Runx1 interaction provides a braking mechanism for hematopoietic differentiation.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23626-23635. doi: 10.1073/pnas.2003228117. Epub 2020 Sep 3. Proc Natl Acad Sci U S A. 2020. PMID: 32883883 Free PMC article.
-
Stat3 Regulates Developmental Hematopoiesis and Impacts Myeloid Cell Function via Canonical and Non-Canonical Modalities.J Innate Immun. 2024;16(1):262-282. doi: 10.1159/000538364. Epub 2024 Apr 24. J Innate Immun. 2024. PMID: 38643762 Free PMC article.
-
Zebrafish Cancer Predisposition Models.Front Cell Dev Biol. 2021 Apr 27;9:660069. doi: 10.3389/fcell.2021.660069. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33987182 Free PMC article. Review.
-
Hematopoietic stem cells, hematopoiesis and disease: lessons from the zebrafish model.Genome Med. 2011 Dec 29;3(12):83. doi: 10.1186/gm299. Genome Med. 2011. PMID: 22206610 Free PMC article.
References
-
- Sun W, Downing JR. Haploinsufficiency of AML1 results in a decrease in the number of LTR-HSCs while simultaneously inducing an increase in more mature progenitors. Blood. 2004;104(12):3565–3572. - PubMed
-
- Ichikawa M, Goyama S, Asai T, et al. AML1/Runx1 negatively regulates quiescent hematopoietic stem cells in adult hematopoiesis. J Immunol. 2008;180(7):4402–4408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

