Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function
- PMID: 20154218
- PMCID: PMC2858485
- DOI: 10.1182/blood-2009-09-246173
Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function
Abstract
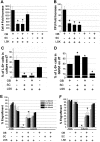
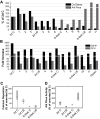
Hematopoietic stem (HSC) and progenitor (HPC) cell fate is governed by intrinsic and extrinsic parameters. We examined the impact of hematopoietic niche elements on HSC and HPC function by analyzing the combined effect of osteoblasts (OBs) and stromal cells (SCs) on Lineage(-)Sca-1(+)CD117(+) (LSK) cells. CFU expansion and marrow repopulating potential of cultured Lineage(-)Sca-1(+)CD117(+) cells were significantly higher in OB compared with SC cultures, thus corroborating the importance of OBs in the competence of the hematopoietic niche. OB-mediated enhancement of HSC and HPC function was reduced in cocultures of OBs and SCs, suggesting that SCs suppressed the OB-mediated hematopoiesis-enhancing activity. Although the suppressive effect of SC was mediated by adipocytes, probably through up-regulation of neuropilin-1, the OB-mediated enhanced hematopoiesis function was elaborated through Notch signaling. Expression of Notch 2, Jagged 1 and 2, Delta 1 and 4, Hes 1 and 5, and Deltex was increased in OB cultures and suppressed in SC and OB/SC cultures. Phenotypic fractionation of OBs did not segregate the hematopoiesis-enhancing activity but demonstrated that this function is common to OBs from different anatomic sites. These data illustrate that OBs promote in vitro maintenance of hematopoietic functions, including repopulating potential by up-regulating Notch-mediated signaling between HSCs and OBs.
Figures
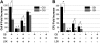
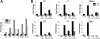
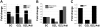
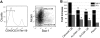
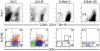
References
-
- Raaijmakers MH, Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol. 2008;15(4):301–306. - PubMed
-
- Haylock DN, Williams B, Johnston HM, et al. Hemopoietic stem cells with higher hemopoietic potential reside at the bone marrow endosteum. Stem Cells. 2007;25(4):1062–1069. - PubMed
-
- Marusić A, Kalinowski JF, Jastrzebski S, Lorenzo JA. Production of leukemia inhibitory factor mRNA and protein by malignant and immortalized bone cells. J Bone Miner Res. 1993;8(5):617–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

