Perturbed hematopoiesis in the Tc1 mouse model of Down syndrome
- PMID: 20154221
- PMCID: PMC2854435
- DOI: 10.1182/blood-2009-06-227629
Perturbed hematopoiesis in the Tc1 mouse model of Down syndrome
Abstract
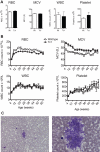
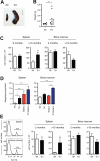
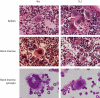
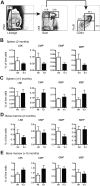
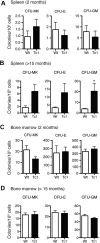
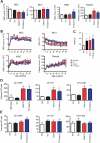
Trisomy of human chromosome 21 (Hsa21) results in Down syndrome (DS), a disorder that affects many aspects of physiology, including hematopoiesis. DS children have greatly increased rates of acute lymphoblastic leukemia and acute megakaryoblastic leukemia (AMKL); DS newborns present with transient myeloproliferative disorder (TMD), a preleukemic form of AMKL. TMD and DS-AMKL almost always carry an acquired mutation in GATA1 resulting in exclusive synthesis of a truncated protein (GATA1s), suggesting that both trisomy 21 and GATA1 mutations are required for leukemogenesis. To gain further understanding of how Hsa21 contributes to hematopoietic abnormalities, we examined the Tc1 mouse model of DS, which carries an almost complete freely segregating copy of Hsa21, and is the most complete model of DS available. We show that although Tc1 mice do not develop leukemia, they have macrocytic anemia and increased extramedullary hematopoiesis. Introduction of GATA1s into Tc1 mice resulted in a synergistic increase in megakaryopoiesis, but did not result in leukemia or a TMD-like phenotype, demonstrating that GATA1s and trisomy of approximately 80% of Hsa21 perturb megakaryopoiesis but are insufficient to induce leukemia.
Figures

References
-
- Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. - PubMed
-
- Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with Down syndrome: data from a multihospital healthcare system. Am J Med Genet A. 2007;143(1):42–50. - PubMed
-
- Hord JD, Gay JC, Whitlock JA. Thrombocytopenia in neonates with trisomy 21. Arch Pediatr Adolesc Med. 1995;149(7):824–825. - PubMed
-
- Miller M, Cosgriff JM. Hematological abnormalities in newborn infants with Down syndrome. Am J Med Genet. 1983;16(2):173–177. - PubMed
-
- Roizen NJ, Amarose AP. Hematologic abnormalities in children with Down syndrome. Am J Med Genet. 1993;46(5):510–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

