Novel bradykinin signaling in adult rat cardiac myocytes through activation of p21-activated kinase
- PMID: 20154261
- PMCID: PMC2853422
- DOI: 10.1152/ajpheart.01070.2009
Novel bradykinin signaling in adult rat cardiac myocytes through activation of p21-activated kinase
Abstract
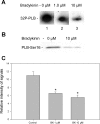
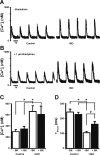
Although bradykinin (BK) is known to exert effects on the myocardium, its intracellular signaling pathways remain poorly understood. Experiments in other cell types indicated that p21-activated kinase-1 (Pak1), a Ser/Thr kinase downstream of small monomeric G proteins, is activated by BK. We previously reported that the expression of active Pak1 in adult cardiac myocytes induced activation of protein phosphatase 2A and dephosphorylation of myofilament proteins (Ke et al. Circ Res 94: 194-200, 2004). In experiments reported here, we tested the hypothesis that BK signals altered protein phosphorylation in adult rat cardiac myocytes through the activation and translocation of Pak1. Treatment of myocytes with BK resulted in the activation of Pak1 as demonstrated by increased autophosphorylation at Thr423 and a diminished striated localization, which is present in the basal state. BK induced dephosphorylation of both cardiac troponin I and phospholamban. Treatment of isolated myocytes with BK also blunted the effect of isoproterenol to enhance peak Ca(2+) and relaxation of Ca(2+) transients. Protein phosphatase 2A was demonstrated to associate with both Pak 1 and phospholamban. Our studies indicate a novel signaling mechanism for BK in adult rat cardiac myocytes and support our hypothesis that Pak 1 is a significant regulator of phosphatase activity in the heart.
Figures




References
-
- Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol 289: H2183–H2192, 2005 - PubMed
-
- Barr E, Carroll J, Kalynych AM, Tripathy SK, Kozarsky K, Wilson JM, Leiden JM. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther 1: 51–58, 1994 - PubMed
-
- Chen J, Parsons S, Brautigan DL. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem 269: 7957–7962, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

