Ac-SDKP inhibits transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts
- PMID: 20154264
- PMCID: PMC2867434
- DOI: 10.1152/ajpheart.00464.2009
Ac-SDKP inhibits transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts
Abstract
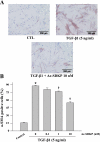
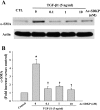
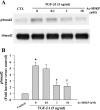
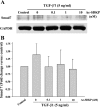
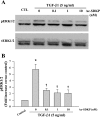
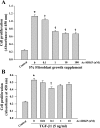
N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) inhibits collagen production and cell proliferation in cultured rat cardiac fibroblasts, but its effect on differentiation of fibroblasts into myofibroblasts is not known. High amounts of transforming growth factor-beta1 (TGF-beta1) have been found in fibrotic cardiac tissue. TGF-beta1 converts fibroblasts into myofibroblasts, which produce more extracellular matrix proteins than fibroblasts. We hypothesized that 1) Ac-SDKP inhibits TGF-beta1-induced differentiation of fibroblasts into myofibroblasts; and 2) this effect is mediated in part by blocking phosphorylation of small-mothers-against-decapentaplegic (Smad) 2 and extracellular signal-regulated kinase (ERK) 1/2. For this study, we used human fetal cardiac fibroblasts (HCFs), which do not spontaneously become myofibroblasts when cultured at low passages. We investigated the effect of Ac-SDKP on TGF-beta1-induced HCF transformation into myofibroblasts, Smad2 and ERK1/2 phosphorylation, Smad7 expression, cell proliferation, and collagen production. We also investigated TGF-beta1 production by HCFs stimulated with endothelin-1 (ET-1). As expected, HCFs treated with TGF-beta1 transformed into myofibroblasts as indicated by increased expression of alpha-smooth muscle actin and a higher proportion of the embryonic isoform of smooth muscle myosin compared with untreated cells. TGF-beta1 also increased Smad2 and ERK1/2 phosphorylation but did not affect Smad7 expression. In addition, TGF-beta1 stimulated HCF proliferation as indicated by an increase in mitochondrial dehydrogenase activity and collagen production (hydroxyproline assay). Ac-SDKP significantly inhibited all of the effects of TGF-beta1. It also inhibited ET-1-stimulated TGF-beta1 production. We concluded that Ac-SDKP markedly suppresses differentiation of human cardiac fibroblasts into myofibroblasts, probably by inhibiting the TGF-beta/Smad/ERK1/2 signaling pathway, and thus mediating its anti-fibrotic effects.
Figures

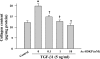
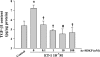
References
-
- Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 - PubMed
-
- Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor β(1)heterozygous mice. J Mol Cell Cardiol 32: 187–195, 2000 - PubMed
-
- Calderone A, Murphy RJ, Lavoie J, Colombo F, Beliveau L. TGF-β1 and prepro-ANP mRNAs are differentially regulated in exercise-induced cardiac hypertrophy. J Appl Physiol 91: 771–776, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

