Human CD4- 8- T cells are a distinctive immunoregulatory subset
- PMID: 20154266
- PMCID: PMC2887271
- DOI: 10.1096/fj.09-153148
Human CD4- 8- T cells are a distinctive immunoregulatory subset
Abstract
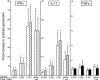
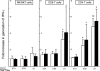
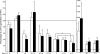
Human CD4(-)8(-) T cells are a minor subset quantitatively but potentially important in immunity because they are predominantly distributed at body surfaces, and their number and activities increase in autoimmune diseases and decrease with aging. Distinguishing characteristics of CD4(-)8(-) T cells are found to include a unique profile of cytokines, including Serpin E1, which is not generated by other T cells, MIF, and TGF-beta. At 2-5% of the total in mixtures with CD4 + CD8 T cells, CD4(-)8(-) T cells enhance the generation of IFN-gamma and IL-17 by up to 12- and 5-fold, respectively, without contributing either cytokine or affecting cytokine production by NK/NKT cells. CD4(-)8(-) T cell-derived MIF is their major enhancer and TGFbeta their principal inhibitor of CD4 and CD8 T cell cytokine production. Decreases in CD4(-)8(-) T cell effects may diminish protective immunity in aging, whereas increases may augment the severity of autoimmune diseases.
Figures
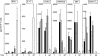

Similar articles
-
IL-21-Induced MHC Class II+ NK Cells Promote the Expansion of Human Uncommitted CD4+ Central Memory T Cells in a Macrophage Migration Inhibitory Factor-Dependent Manner.J Immunol. 2016 Jul 1;197(1):85-96. doi: 10.4049/jimmunol.1501147. Epub 2016 May 27. J Immunol. 2016. PMID: 27233967
-
The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets.J Leukoc Biol. 2003 Oct;74(4):471-8. doi: 10.1189/jlb.0503228. J Leukoc Biol. 2003. PMID: 14519757 Free PMC article. Review.
-
Patterns of CD4/CD8 T-cell ratio in dialysis effluents predict the long-term outcome of peritonitis in patients undergoing peritoneal dialysis.Nephrol Dial Transplant. 2003 Jun;18(6):1181-9. doi: 10.1093/ndt/gfg079. Nephrol Dial Transplant. 2003. PMID: 12748353
-
Effect of long-term anti-CD4 or anti-CD8 treatment on the development of lpr CD4- CD8- double negative T cells and of the autoimmune syndrome in MRL-lpr/lpr mice.J Autoimmun. 1995 Feb;8(1):33-45. doi: 10.1006/jaut.1995.0003. J Autoimmun. 1995. PMID: 7734035
-
CD4/CD8-negative T cells with alpha beta antigen receptors.Curr Opin Immunol. 1994 Jun;6(3):438-41. doi: 10.1016/0952-7915(94)90124-4. Curr Opin Immunol. 1994. PMID: 7917112 Review.
Cited by
-
Adult Renal Stem/Progenitor Cells Can Modulate T Regulatory Cells and Double Negative T Cells.Int J Mol Sci. 2020 Dec 29;22(1):274. doi: 10.3390/ijms22010274. Int J Mol Sci. 2020. PMID: 33383950 Free PMC article.
-
Preferential enhancement of older human T cell cytokine generation, chemotaxis, proliferation and survival by lenalidomide.Clin Immunol. 2011 Feb;138(2):201-11. doi: 10.1016/j.clim.2010.11.002. Epub 2010 Dec 3. Clin Immunol. 2011. PMID: 21130040 Free PMC article.
-
Defective T cell chemotaxis to sphingosine 1-phosphate and chemokine CCL21 in idiopathic T lymphocytopenia.J Clin Immunol. 2011 Oct;31(5):744-51. doi: 10.1007/s10875-011-9554-2. Epub 2011 Jun 14. J Clin Immunol. 2011. PMID: 21671128
-
Lenalidomide enhancement of human T cell functions in human immunodeficiency virus (HIV)-infected and HIV-negative CD4 T lymphocytopenic patients.Clin Exp Immunol. 2012 Aug;169(2):182-9. doi: 10.1111/j.1365-2249.2012.04603.x. Clin Exp Immunol. 2012. PMID: 22774993 Free PMC article.
-
Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane.PLoS One. 2017 Jun 30;12(6):e0179012. doi: 10.1371/journal.pone.0179012. eCollection 2017. PLoS One. 2017. PMID: 28666020 Free PMC article.
References
-
- Koyamada N, Ohteki T, Abo T, Fukumori T, Ohkouchi N, Satomi S, Taguchi Y, Kusumi A, Mori S, Kumagai K. Induction of specific tolerance by hepatic double-negative CD4−8− alpha beta T cells of mice immunized with allogeneic cells via the portal vein in vivo [corrected] Cell Immunol. 1993;149:107–116. - PubMed
-
- Pawankar R, Okuda M. A comparative study of the characteristics of intraepithelial and lamina propria lymphocytes of the human nasal mucosa. Allergy. 1993;48:99–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

