Novel myelin penta- and hexa-acetyl-galactosyl-ceramides: structural characterization and immunoreactivity in cerebrospinal fluid
- PMID: 20154333
- PMCID: PMC3035502
- DOI: 10.1194/jlr.M001396
Novel myelin penta- and hexa-acetyl-galactosyl-ceramides: structural characterization and immunoreactivity in cerebrospinal fluid
Abstract
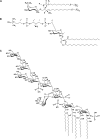
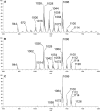
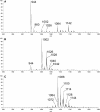
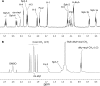
Fast migrating cerebrosides (FMC) are derivatives of galactosylceramide (GalCer). The structures of the most hydrophobic FMC-5, FMC-6, and FMC-7 were determined by electrospray ionization linear ion-trap mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy complementing previous NMR spectroscopy and gas chromatography-mass spectrometry to be 3-O-acetyl-sphingosine-GalCer derivatives with galactose O-acetyl modifications. FMC-5 and FMC-6 are 3-O-acetyl-sphingosine-2,3,4,6-tetra-O-acetyl-GalCer with nonhydroxy and hydroxy-N-fatty-acids, while FMC-7 has an additional O-acetylation of the 2-hydroxy-fatty acid. The immuno-reactivity in human cerebrospinal fluid (CSF) to these acetylated glycolipids was examined in central nervous system (CNS) infectious disease, noninflammatory disorders, and multiple sclerosis (MS). Screening for lipid binding in MS and other neurological disease groups revealed that the greatest anti-hydrophobic FMC reactivity was observed in the inflammatory CNS diseases (meningitis, meningo-encephalitis, and subacute sclerosing panencephalitis). Some MS patients had increased reactivity with the hydrophobic FMCs and with glycoglycerophospholipid MfGL-II from Mycoplasma fermentans. The cross-reactivity of highly acetylated GalCer with microbial acyl-glycolipid raises the possibility that myelin-O-acetyl-cerebrosides, bacterial infection, and neurological disease are linked.
Figures


Similar articles
-
3-O-acetyl-sphingosine-series myelin glycolipids: characterization of novel 3-O-acetyl-sphingosine galactosylceramide.J Lipid Res. 2002 May;43(5):751-61. J Lipid Res. 2002. PMID: 11971946
-
Characterization of novel myelin components 3-O-acetyl-sphingosine galactosylceramides by electrospray ionization Q-TOF MS and MS/CID-MS of Li+ adducts.J Mass Spectrom. 2007 May;42(5):598-620. doi: 10.1002/jms.1190. J Mass Spectrom. 2007. PMID: 17370250
-
The structural and functional role of myelin fast-migrating cerebrosides: pathological importance in multiple sclerosis.Clin Lipidol. 2011 Apr;6(2):159-179. doi: 10.2217/clp.11.8. Clin Lipidol. 2011. PMID: 22701512 Free PMC article.
-
Tailored design of NKT-stimulatory glycolipids for polarization of immune responses.J Biomed Sci. 2017 Mar 23;24(1):22. doi: 10.1186/s12929-017-0325-0. J Biomed Sci. 2017. PMID: 28335781 Free PMC article. Review.
-
Immune responses to myelin antigens in multiple sclerosis.Ann N Y Acad Sci. 1984;436:221-30. doi: 10.1111/j.1749-6632.1984.tb14793.x. Ann N Y Acad Sci. 1984. PMID: 6085227 Review.
Cited by
-
Invariant natural killer T cells and their ligands: focus on multiple sclerosis.Immunology. 2015 Aug;145(4):468-75. doi: 10.1111/imm.12481. Epub 2015 Jun 25. Immunology. 2015. PMID: 25976210 Free PMC article. Review.
-
New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration.Int J Mol Sci. 2021 Jul 7;22(14):7319. doi: 10.3390/ijms22147319. Int J Mol Sci. 2021. PMID: 34298940 Free PMC article. Review.
-
Recent advances in the mass spectrometric analysis of glycosphingolipidome - A review.Anal Chim Acta. 2020 Oct 2;1132:134-155. doi: 10.1016/j.aca.2020.05.051. Epub 2020 May 24. Anal Chim Acta. 2020. PMID: 32980104 Free PMC article. Review.
-
Don't Be Surprised When These Surprise You: Some Infrequently Studied Sphingoid Bases, Metabolites, and Factors That Should Be Kept in Mind During Sphingolipidomic Studies.Int J Mol Sci. 2025 Jan 14;26(2):650. doi: 10.3390/ijms26020650. Int J Mol Sci. 2025. PMID: 39859363 Free PMC article. Review.
-
Sphingolipid Players in Multiple Sclerosis: Their Influence on the Initiation and Course of the Disease.Int J Mol Sci. 2022 May 10;23(10):5330. doi: 10.3390/ijms23105330. Int J Mol Sci. 2022. PMID: 35628142 Free PMC article. Review.
References
-
- Dasgupta S., Levery S. B., Hogan E. L. 2002. 3-O-acetyl-sphingosine-series myelin glycolipids: characterization of novel 3-O-acetyl-sphingosine galactosylceramide. J. Lipid Res. 43: 751–761. - PubMed
-
- Bennion B., Dasgupta S., Hogan E. L., Levery S. B. 2007. Characterization of novel myelin components 3-O-acetyl-sphingosine galactosylceramides by electrospray ionization Q-TOF MS and MS/CID-MS of Li+ adducts. J. Mass Spectrom. 42: 598–620. - PubMed
-
- Katzenellenbogen E., Kocharova N. A., Korzeniowska-Kowal A., Gamian A., Bogulska M., Szostko B., Shashkov A. S., Knirel Y. A. 2008. Immunochemical studies of the lipopolysaccharides of Hafnia alvei PCM 1219 and other strains with the O-antigens containing D-glucose 1-phosphate and 2-deoxy-2-[(R)-3-hydroxybutyramido]-D-glucose. Arch. Immunol. Ther. Exp. (Warsz.). 56: 347–352. - PMC - PubMed
-
- Parolis H., Parolis L. A., Olivieri G. 1997. Structural studies on the Shigella-like Escherichia coli O121 O-specific polysaccharide. Carbohydr. Res. 303: 319–325. - PubMed
-
- Ali T., Weintraub A., Widmalm G. 2006. Structural determination of the O-antigenic polysaccharide from the Shiga toxin-producing Escherichia coli O171. Carbohydr. Res. 341: 1878–1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

