Proteome analysis of the penicillin producer Penicillium chrysogenum: characterization of protein changes during the industrial strain improvement
- PMID: 20154335
- PMCID: PMC2877979
- DOI: 10.1074/mcp.M900327-MCP200
Proteome analysis of the penicillin producer Penicillium chrysogenum: characterization of protein changes during the industrial strain improvement
Abstract
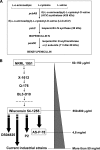
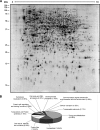
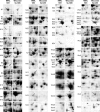
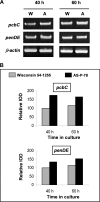
Proteomics is a powerful tool to understand the molecular mechanisms causing the production of high penicillin titers by industrial strains of the filamentous fungus Penicillium chrysogenum as the result of strain improvement programs. Penicillin biosynthesis is an excellent model system for many other bioactive microbial metabolites. The recent publication of the P. chrysogenum genome has established the basis to understand the molecular processes underlying penicillin overproduction. We report here the proteome reference map of P. chrysogenum Wisconsin 54-1255 (the genome project reference strain) together with an in-depth study of the changes produced in three different strains of this filamentous fungus during industrial strain improvement. Two-dimensional gel electrophoresis, peptide mass fingerprinting, and tandem mass spectrometry were used for protein identification. Around 1000 spots were visualized by "blue silver" colloidal Coomassie staining in a non-linear pI range from 3 to 10 with high resolution, which allowed the identification of 950 proteins (549 different proteins and isoforms). Comparison among the cytosolic proteomes of the wild-type NRRL 1951, Wisconsin 54-1255 (an improved, moderate penicillin producer), and AS-P-78 (a penicillin high producer) strains indicated that global metabolic reorganizations occurred during the strain improvement program. The main changes observed in the high producer strains were increases of cysteine biosynthesis (a penicillin precursor), enzymes of the pentose phosphate pathway, and stress response proteins together with a reduction in virulence and in the biosynthesis of other secondary metabolites different from penicillin (pigments and isoflavonoids). In the wild-type strain, we identified enzymes to utilize cellulose, sorbitol, and other carbon sources that have been lost in the high penicillin producer strains. Changes in the levels of a few specific proteins correlated well with the improved penicillin biosynthesis in the high producer strains. These results provide useful information to improve the production of many other bioactive secondary metabolites.
Figures




Similar articles
-
The Penicillium chrysogenum extracellular proteome. Conversion from a food-rotting strain to a versatile cell factory for white biotechnology.Mol Cell Proteomics. 2010 Dec;9(12):2729-44. doi: 10.1074/mcp.M110.001412. Epub 2010 Sep 7. Mol Cell Proteomics. 2010. PMID: 20823121 Free PMC article.
-
Key role of LaeA and velvet complex proteins on expression of β-lactam and PR-toxin genes in Penicillium chrysogenum: cross-talk regulation of secondary metabolite pathways.J Ind Microbiol Biotechnol. 2017 May;44(4-5):525-535. doi: 10.1007/s10295-016-1830-y. Epub 2016 Aug 26. J Ind Microbiol Biotechnol. 2017. PMID: 27565675 Review.
-
Catabolism of phenylacetic acid in Penicillium rubens. Proteome-wide analysis in response to the benzylpenicillin side chain precursor.J Proteomics. 2018 Sep 15;187:243-259. doi: 10.1016/j.jprot.2018.08.006. Epub 2018 Aug 6. J Proteomics. 2018. PMID: 30092379
-
Penicillium chrysogenum: Beyond the penicillin.Adv Appl Microbiol. 2024;127:143-221. doi: 10.1016/bs.aambs.2024.02.006. Epub 2024 Apr 16. Adv Appl Microbiol. 2024. PMID: 38763527 Review.
-
PcFKH1, a novel regulatory factor from the forkhead family, controls the biosynthesis of penicillin in Penicillium chrysogenum.Biochimie. 2015 Aug;115:162-76. doi: 10.1016/j.biochi.2015.05.015. Epub 2015 Jun 4. Biochimie. 2015. PMID: 26049046
Cited by
-
Impact of Classical Strain Improvement of Penicillium rubens on Amino Acid Metabolism during β-Lactam Production.Appl Environ Microbiol. 2020 Jan 21;86(3):e01561-19. doi: 10.1128/AEM.01561-19. Print 2020 Jan 21. Appl Environ Microbiol. 2020. PMID: 31757830 Free PMC article.
-
Biosynthetic Incorporation of Site-Specific Isotopes in β-Lactam Antibiotics Enables Biophysical Studies.ACS Chem Biol. 2020 May 15;15(5):1148-1153. doi: 10.1021/acschembio.9b01054. Epub 2020 Mar 20. ACS Chem Biol. 2020. PMID: 32175720 Free PMC article.
-
New Antimicrobial Potential and Structural Properties of PAFB: A Cationic, Cysteine-Rich Protein from Penicillium chrysogenum Q176.Sci Rep. 2018 Jan 29;8(1):1751. doi: 10.1038/s41598-018-20002-2. Sci Rep. 2018. PMID: 29379111 Free PMC article.
-
Cephalosporin C biosynthesis and fermentation in Acremonium chrysogenum.Appl Microbiol Biotechnol. 2022 Oct;106(19-20):6413-6426. doi: 10.1007/s00253-022-12181-w. Epub 2022 Sep 17. Appl Microbiol Biotechnol. 2022. PMID: 36114850 Review.
-
bZIP transcription factors PcYap1 and PcRsmA link oxidative stress response to secondary metabolism and development in Penicillium chrysogenum.Microb Cell Fact. 2022 Apr 2;21(1):50. doi: 10.1186/s12934-022-01765-w. Microb Cell Fact. 2022. PMID: 35366869 Free PMC article.
References
-
- Liras P., Martín J. F. (2009) β-Lactam antibiotics, in Encyclopedia of Microbiology (Schaechter M. ed) 3rd Ed., pp. 274–289, Elsevier Ltd., Oxford
-
- García-Rico R. O., Fierro F., Mauriz E., Gómez A., Fernández-Bodega M. A., Martín J. F. (2008) The heterotrimeric Gα protein Pga1 regulates biosynthesis of penicillin, chrysogenin and roquefortine in Penicillium chrysogenum. Microbiology 154, 3567–3578 - PubMed
-
- Fleming A. (1929) On the antibacterial action of cultures of a penicillium with special reference to their use in the isolation of B. influenza. Br. J. Exp. Pathol 10, 226–236 - PubMed
-
- Elander R. P. (1983) Strain improvement and preservation of beta-lactam producing microorganisms, in Antibiotics Containing the Beta-lactam Structure I (Demain A. L., Solomon N. eds) pp. 97–146, Springer-Verlag, New York
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

