Functional conservation of human Spastin in a Drosophila model of autosomal dominant-hereditary spastic paraplegia
- PMID: 20154342
- PMCID: PMC2860889
- DOI: 10.1093/hmg/ddq064
Functional conservation of human Spastin in a Drosophila model of autosomal dominant-hereditary spastic paraplegia
Abstract
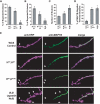
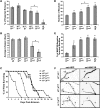
Mutations in spastin are the most frequent cause of the neurodegenerative disease autosomal dominant-hereditary spastic paraplegia (AD-HSP). Drosophila melanogaster lacking spastin exhibit striking behavioral similarities to human patients suffering from AD-HSP, suggesting conservation of Spastin function between the species. Consistent with this, we show that exogenous expression of wild-type Drosophila or human spastin rescues behavioral and cellular defects in spastin null flies equivalently. This enabled us to generate genetically representative models of AD-HSP, which arises from dominant mutations in spastin rather than a complete loss of the gene. Flies co-expressing one copy of wild-type human spastin and one encoding the K388R catalytic domain mutation in the fly spastin null background, exhibit aberrant distal synapse morphology and microtubule distribution, similar to but less severe than spastin nulls. R388 or a separate nonsense mutation act dominantly and are furthermore sufficient to confer partial rescue, supporting in vitro evidence for additional, non-catalytic Spastin functions. Using this model, we tested the observation from human pedigrees that S44L and P45Q are trans-acting modifiers of mutations affecting the Spastin catalytic domain. As in humans, both L44 and Q45 are largely silent when heterozygous, but exacerbate mutant phenotypes when expressed in trans with R388. These transgenic 'AD-HSP' flies therefore provide a powerful and tractable model to enhance our understanding of the cellular and behavioral consequences of human spastin mutations and test hypotheses directly relevant to the human disease.
Figures



Similar articles
-
Cold temperature improves mobility and survival in Drosophila models of autosomal-dominant hereditary spastic paraplegia (AD-HSP).Dis Model Mech. 2014 Aug;7(8):1005-12. doi: 10.1242/dmm.013987. Epub 2014 Jun 6. Dis Model Mech. 2014. PMID: 24906373 Free PMC article.
-
Intragenic modifiers of hereditary spastic paraplegia due to spastin gene mutations.Neurogenetics. 2004 Sep;5(3):157-64. doi: 10.1007/s10048-004-0186-z. Epub 2004 Jul 10. Neurogenetics. 2004. PMID: 15248095
-
Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine.J Clin Invest. 2005 Nov;115(11):3026-34. doi: 10.1172/JCI24694. J Clin Invest. 2005. PMID: 16276413 Free PMC article.
-
[From gene to disease; spastin and hereditary spastic paraparesis].Ned Tijdschr Geneeskd. 2004 Jan 24;148(4):179-81. Ned Tijdschr Geneeskd. 2004. PMID: 14974310 Review. Dutch.
-
Hereditary spastic paraplegia SPG4: what is known and not known about the disease.Brain. 2015 Sep;138(Pt 9):2471-84. doi: 10.1093/brain/awv178. Epub 2015 Jun 20. Brain. 2015. PMID: 26094131 Free PMC article. Review.
Cited by
-
Regulation of apical constriction via microtubule- and Rab11-dependent apical transport during tissue invagination.Mol Biol Cell. 2021 May 1;32(10):1033-1047. doi: 10.1091/mbc.E21-01-0021. Epub 2021 Mar 31. Mol Biol Cell. 2021. PMID: 33788621 Free PMC article.
-
Microtubule-targeting drugs rescue axonal swellings in cortical neurons from spastin knockout mice.Dis Model Mech. 2013 Jan;6(1):72-83. doi: 10.1242/dmm.008946. Epub 2012 Jul 5. Dis Model Mech. 2013. PMID: 22773755 Free PMC article.
-
The Presynaptic Microtubule Cytoskeleton in Physiological and Pathological Conditions: Lessons from Drosophila Fragile X Syndrome and Hereditary Spastic Paraplegias.Front Mol Neurosci. 2016 Jul 25;9:60. doi: 10.3389/fnmol.2016.00060. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27504085 Free PMC article. Review.
-
Normal spastin gene dosage is specifically required for axon regeneration.Cell Rep. 2012 Nov 29;2(5):1340-50. doi: 10.1016/j.celrep.2012.09.032. Epub 2012 Nov 1. Cell Rep. 2012. PMID: 23122959 Free PMC article.
-
Loss of Drosophila melanogaster p21-activated kinase 3 suppresses defects in synapse structure and function caused by spastin mutations.Genetics. 2011 Sep;189(1):123-35. doi: 10.1534/genetics.111.130831. Epub 2011 Jul 29. Genetics. 2011. PMID: 21705760 Free PMC article.
References
-
- Fink J.K. Hereditary spastic paraplegia. Neurol. Clin. 2002;20:711–726. - PubMed
-
- Beetz C., Nygren A., Schickel J., Auer-Grumbach M., Burk K., Heide G., Kassubek J., Klimpe S., Klopstock T., Kreuz F., et al. High frequency of partial SPAST deletions in autosomal dominant hereditary spastic paraplegia. Neurology. 2006;67:1926–1930. - PubMed
-
- Fink J.K., Rainier S. Hereditary spastic paraplegia: spastin phenotype and function. Arch. Neurol. 2004;61:830–833. - PubMed
-
- Salinas S., Carazo-Salas R.E., Proukakis C., Schiavo G., Warner T.T. Spastin and microtubules: Functions in health and disease. J. Neurosci. Res. 2007;85:2778–2782. - PubMed
-
- Roll-Mecak A., Vale R.D. The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr. Biol. 2005;15:650–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

