The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory
- PMID: 20154587
- PMCID: PMC2841791
- DOI: 10.1097/PAS.0b013e3181cf3d79
The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory
Abstract
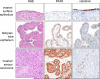
Ovarian cancer is the most lethal gynecologic malignancy. Efforts at early detection and new therapeutic approaches to reduce mortality have been largely unsuccessful, because the origin and pathogenesis of epithelial ovarian cancer are poorly understood. Despite numerous studies that have carefully scrutinized the ovaries for precursor lesions, none have been found. This has led to the proposal that ovarian cancer develops de novo. Studies have shown that epithelial ovarian cancer is not a single disease but is composed of a diverse group of tumors that can be classified based on distinctive morphologic and molecular genetic features. One group of tumors, designated type I, is composed of low-grade serous, low-grade endometrioid, clear cell, mucinous and transitional (Brenner) carcinomas. These tumors generally behave in an indolent fashion, are confined to the ovary at presentation and, as a group, are relatively genetically stable. They lack mutations of TP53, but each histologic type exhibits a distinctive molecular genetic profile. Moreover, the carcinomas exhibit a shared lineage with the corresponding benign cystic neoplasm, often through an intermediate (borderline tumor) step, supporting the morphologic continuum of tumor progression. In contrast, another group of tumors, designated type II, is highly aggressive, evolves rapidly and almost always presents in advanced stage. Type II tumors include conventional high-grade serous carcinoma, undifferentiated carcinoma, and malignant mixed mesodermal tumors (carcinosarcoma). They displayTP53 mutations in over 80% of cases and rarely harbor the mutations that are found in the type I tumors. Recent studies have also provided cogent evidence that what have been traditionally thought to be primary ovarian tumors actually originate in other pelvic organs and involve the ovary secondarily. Thus, it has been proposed that serous tumors arise from the implantation of epithelium (benign or malignant) from the fallopian tube. Endometrioid and clear cell tumors have been associated with endometriosis that is regarded as the precursor of these tumors. As it is generally accepted that endometriosis develops from endometrial tissue by retrograde menstruation, it is reasonable to assume that the endometrium is the source of these ovarian neoplasms. Finally, preliminary data suggest that mucinous and transitional (Brenner) tumors arise from transitional-type epithelial nests at the tubal-mesothelial junction by a process of metaplasia. Appreciation of these new concepts will allow for a more rationale approach to screening, treatment, and prevention that potentially can have a significant impact on reducing the mortality of this devastating disease.
Figures


References
-
- Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859–64. - PubMed
-
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. - PubMed
-
- Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–90. - PubMed
-
- Carcangiu ML, Radice P, Manoukian S, et al. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol. 2004;23:35–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

