Therapeutic accessibility of caspase-mediated cell death as a key pathomechanism in indirect acute lung injury
- PMID: 20154604
- PMCID: PMC3502073
- DOI: 10.1097/CCM.0b013e3181d4563f
Therapeutic accessibility of caspase-mediated cell death as a key pathomechanism in indirect acute lung injury
Abstract
Objective: Indirect acute lung injury is associated with high morbidity and mortality. However, the underlying pathophysiology is only marginally understood, and so far no pathophysiologic-based remedy exists. We hypothesized that apoptosis of lung epithelial cells is a pathophysiological relevant process in the development of indirect acute lung injury and that it should be accessible to a siRNA-based therapeutic intervention in vivo.
Design: Prospective, randomized, controlled animal study.
Setting: Basic science laboratory of a university affiliated level one trauma center.
Subjects: Male C3H/HeN mice, 8 wks old, n = 121.
Interventions: First, siRNA sequences to knock-down caspase-3 expression at a RNA and protein level were evaluated in vitro. Then, C3H/HeN mice were subjected to hemorrhagic shock, after which they received either a caspase-3 siRNA or a control/nonsense siRNA. Subsequently, they were then subjected to polymicrobial sepsis (induced by cecal ligation and puncture).
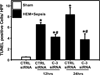
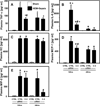
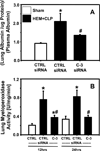
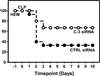
Measurements and main results: Twelve and 24 hrs after sepsis, increased lung epithelial apoptosis was observed, as evidenced by active caspase-3 Western blotting, caspase-3, TUNEL-, and M-30 immunohistochemistry. Hallmarks of acute lung injury, such as increased concentrations of pulmonary cytokines/chemokines, lung protein leakage, myeloperoxidase activity, and altered lung histology, were evident in response to these insults. The single intratracheal instillation of caspase-3 siRNA not only attenuated lung apoptosis and inflammation but also ameliorated the development of acute lung injury in treated mice. Most interestingly, this experimental therapeutic approach markedly improved 10-day survival of hemorrhaged septic mice.
Conclusions: Apoptosis of lung epithelial cells is a relevant pathomechanism in the development of hemorrhage-induced indirect septic acute lung injury, and caspase-3 appears to be a valuable therapeutic target accessible by siRNA treatment in vivo.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures



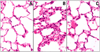
Comment in
-
Intratracheal siRNA for the in vivo silencing of caspase-3: a novel therapy for acute lung injury?Crit Care Med. 2010 Apr;38(4):1223-4. doi: 10.1097/CCM.0b013e3181cfb46a. Crit Care Med. 2010. PMID: 20335707 No abstract available.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. - PubMed
-
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. - PubMed
-
- Herridge MS, Angus DC. Acute lung injury--affecting many lives. N Engl J Med. 2005;353:1736–1738. - PubMed
-
- Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. - PubMed
-
- Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

