Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis
- PMID: 20154707
- PMCID: PMC2916016
- DOI: 10.1038/nsmb.1758
Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis
Abstract
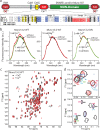
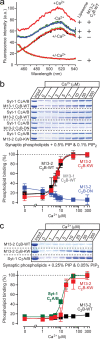
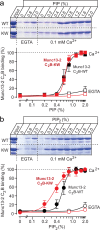
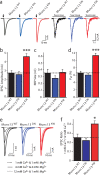
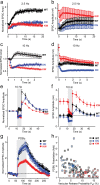
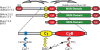
Munc13 is a multidomain protein present in presynaptic active zones that mediates the priming and plasticity of synaptic vesicle exocytosis, but the mechanisms involved remain unclear. Here we use biophysical, biochemical and electrophysiological approaches to show that the central C(2)B domain of Munc13 functions as a Ca(2+) regulator of short-term synaptic plasticity. The crystal structure of the C(2)B domain revealed an unusual Ca(2+)-binding site with an amphipathic alpha-helix. This configuration confers onto the C(2)B domain unique Ca(2+)-dependent phospholipid-binding properties that favor phosphatidylinositolphosphates. A mutation that inactivated Ca(2+)-dependent phospholipid binding to the C(2)B domain did not alter neurotransmitter release evoked by isolated action potentials, but it did depress release evoked by action-potential trains. In contrast, a mutation that increased Ca(2+)-dependent phosphatidylinositolbisphosphate binding to the C(2)B domain enhanced release evoked by isolated action potentials and by action-potential trains. Our data suggest that, during repeated action potentials, Ca(2+) and phosphatidylinositolphosphate binding to the Munc13 C(2)B domain potentiate synaptic vesicle exocytosis, thereby offsetting synaptic depression induced by vesicle depletion.
Figures

Similar articles
-
A Hyperactive Form of unc-13 Enhances Ca2+ Sensitivity and Synaptic Vesicle Release Probability in C. elegans.Cell Rep. 2019 Sep 10;28(11):2979-2995.e4. doi: 10.1016/j.celrep.2019.08.018. Cell Rep. 2019. PMID: 31509756 Free PMC article.
-
Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion.J Neurosci. 2007 Jan 31;27(5):1200-10. doi: 10.1523/JNEUROSCI.4908-06.2007. J Neurosci. 2007. PMID: 17267576 Free PMC article.
-
Munc13-1 is a Ca2+-phospholipid-dependent vesicle priming hub that shapes synaptic short-term plasticity and enables sustained neurotransmission.Neuron. 2021 Dec 15;109(24):3980-4000.e7. doi: 10.1016/j.neuron.2021.09.054. Epub 2021 Oct 26. Neuron. 2021. PMID: 34706220 Free PMC article.
-
The presynaptic active zone.Neuron. 2012 Jul 12;75(1):11-25. doi: 10.1016/j.neuron.2012.06.012. Neuron. 2012. PMID: 22794257 Free PMC article. Review.
-
The synaptic vesicle cycle.Annu Rev Neurosci. 2004;27:509-47. doi: 10.1146/annurev.neuro.26.041002.131412. Annu Rev Neurosci. 2004. PMID: 15217342 Review.
Cited by
-
Different priming states of synaptic vesicles underlie distinct release probabilities at hippocampal excitatory synapses.Neuron. 2022 Dec 21;110(24):4144-4161.e7. doi: 10.1016/j.neuron.2022.09.035. Epub 2022 Oct 18. Neuron. 2022. PMID: 36261033 Free PMC article.
-
Role of phosphoinositides at the neuronal synapse.Subcell Biochem. 2012;59:131-75. doi: 10.1007/978-94-007-3015-1_5. Subcell Biochem. 2012. PMID: 22374090 Free PMC article. Review.
-
A molecular mechanism for calcium-mediated synaptotagmin-triggered exocytosis.Nat Struct Mol Biol. 2018 Oct;25(10):911-917. doi: 10.1038/s41594-018-0130-9. Epub 2018 Oct 5. Nat Struct Mol Biol. 2018. PMID: 30291360 Free PMC article.
-
Synaptic Secretion and Beyond: Targeting Synapse and Neurotransmitters to Treat Neurodegenerative Diseases.Oxid Med Cell Longev. 2022 Jul 25;2022:9176923. doi: 10.1155/2022/9176923. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35923862 Free PMC article. Review.
-
CAPS and Munc13 utilize distinct PIP2-linked mechanisms to promote vesicle exocytosis.Mol Biol Cell. 2014 Feb;25(4):508-21. doi: 10.1091/mbc.E12-11-0829. Epub 2013 Dec 19. Mol Biol Cell. 2014. PMID: 24356451 Free PMC article.
References
-
- Katz B. The release of neuronal transmitter substances. Liverpool University Press; Liverpool, UK: 1969.
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. - PubMed
-
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. - PubMed
-
- Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homologues of C. elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 1995;270:25273–25280. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

