A potential tumor suppressor role for Hic1 in breast cancer through transcriptional repression of ephrin-A1
- PMID: 20154726
- PMCID: PMC3025282
- DOI: 10.1038/onc.2010.12
A potential tumor suppressor role for Hic1 in breast cancer through transcriptional repression of ephrin-A1
Abstract
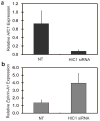
The tumor suppressor gene hypermethylated in cancer 1 (HIC1), which encodes a transcriptional repressor, is epigenetically inactivated in various human cancers. In this study, we show that HIC1 is a direct transcriptional repressor of the gene encoding ephrin-A1, a cell surface ligand implicated in the pathogenesis of epithelial cancers. We also show that mouse embryos lacking both Hic1 alleles manifest developmental defects spatially associated with the misexpression of ephrin-A1, and that overexpression of ephrin-A1 is a feature of tumors arising in Hic1 heterozygous mice in which the remaining wild-type allele is epigenetically silenced. In breast cancer, we find that ephrin-A1 expression is common in vivo, but that in cell culture, expression of the EphA receptors is predominant. Restoration of HIC1 function in breast cancer cells leads to a reduction in tumor growth in vivo, an effect that can be partially rescued by co-overexpression of ephrin-A1. Interestingly, overexpression of ephrin-A1 in vitro triggers downregulation of EphA2 and EphA4 levels, resulting in an expression pattern similar to that seen in vivo. We conclude that Hic1 spatially restricts ephrin-A1 expression in development, and that upregulated expression of ephrin-A1 resulting from epigenetic silencing of HIC1 in cancer cells may be an important mechanism in epithelial malignancy.
Figures

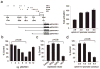



Similar articles
-
The receptor tyrosine kinase EphA2 is a direct target gene of hypermethylated in cancer 1 (HIC1).J Biol Chem. 2012 Feb 17;287(8):5366-78. doi: 10.1074/jbc.M111.329466. Epub 2011 Dec 19. J Biol Chem. 2012. PMID: 22184117 Free PMC article.
-
Ephrin-A1 inhibits NSCLC tumor growth via induction of Cdx-2 a tumor suppressor gene.BMC Cancer. 2012 Jul 23;12:309. doi: 10.1186/1471-2407-12-309. BMC Cancer. 2012. PMID: 22824143 Free PMC article.
-
Implication of HIC1 (Hypermethylated In Cancer 1) in the DNA damage response.Bull Cancer. 2009 Nov;96(11):E66-72. doi: 10.1684/bdc.2009.0959. Bull Cancer. 2009. PMID: 19822477 Review.
-
Hypermethylated in cancer 1 (HIC1) recruits polycomb repressive complex 2 (PRC2) to a subset of its target genes through interaction with human polycomb-like (hPCL) proteins.J Biol Chem. 2012 Mar 23;287(13):10509-10524. doi: 10.1074/jbc.M111.320234. Epub 2012 Feb 7. J Biol Chem. 2012. PMID: 22315224 Free PMC article.
-
[Chromosome arm 17p13.3: could HIC1 be the one ?].Med Sci (Paris). 2006 Jan;22(1):54-61. doi: 10.1051/medsci/200622154. Med Sci (Paris). 2006. PMID: 16386221 Review. French.
Cited by
-
The role of miR-128 in cancer development, prevention, drug resistance, and immunotherapy.Front Oncol. 2023 Jan 19;12:1067974. doi: 10.3389/fonc.2022.1067974. eCollection 2022. Front Oncol. 2023. PMID: 36793341 Free PMC article. Review.
-
The important molecular markers on chromosome 17 and their clinical impact in breast cancer.Int J Mol Sci. 2011;12(9):5672-83. doi: 10.3390/ijms12095672. Epub 2011 Sep 5. Int J Mol Sci. 2011. PMID: 22016618 Free PMC article. Review.
-
GEAMP, a novel gastroesophageal junction carcinoma cell line derived from a malignant pleural effusion.Lab Invest. 2020 Jan;100(1):16-26. doi: 10.1038/s41374-019-0278-x. Epub 2019 Jul 10. Lab Invest. 2020. PMID: 31292541 Free PMC article.
-
Signification of Hypermethylated in Cancer 1 (HIC1) as Tumor Suppressor Gene in Tumor Progression.Cancer Microenviron. 2012 Dec;5(3):285-93. doi: 10.1007/s12307-012-0103-1. Epub 2012 Apr 13. Cancer Microenviron. 2012. PMID: 22528874 Free PMC article.
-
Association between HIC1 promoter methylation and solid tumor: A meta-analysis.EXCLI J. 2020 Apr 7;19:476-489. doi: 10.17179/excli2020-1102. eCollection 2020. EXCLI J. 2020. PMID: 32398971 Free PMC article.
References
-
- Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037–49. - PubMed
-
- Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–24. - PubMed
-
- Carter MG, Johns MA, Zeng X, Zhou L, Zink MC, Mankowski JL, et al. Mice deficient in the candidate tumor suppressor gene Hic1 exhibit developmental defects of structures affected in the Miller-Dieker syndrome. Hum Mol Genet. 2000;9:413–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

