Assessment of immunogenicity of romiplostim in clinical studies with ITP subjects
- PMID: 20155267
- PMCID: PMC2900600
- DOI: 10.1007/s00277-010-0908-2
Assessment of immunogenicity of romiplostim in clinical studies with ITP subjects
Abstract
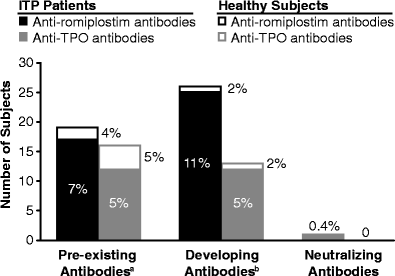
Romiplostim is an Fc-peptide fusion protein that activates intracellular transcriptional pathways via the thrombopoietin (TPO) receptor leading to increased platelet production. Romiplostim has been engineered to have no amino acid sequence homology to endogenous TPO. Recombinant protein therapeutics can be at a risk of development of an antibody response that can impact efficacy and safety. Hence, a strategy to detect potential antibody formation to the drug and to related endogenous molecules can be useful. The immunogenicity assessment strategy involved both the detection and characterization of binding and neutralizing antibodies. The method for detection was based on a surface plasmon resonance biosensor platform using the Biacore 3000. Samples that tested positive for binding antibodies in the Biacore immunoassay were then tested in a neutralization assay. Serum samples from 225 subjects with immune thrombocytopenic purpura (ITP) dosed with romiplostim and 45 ITP subjects dosed with placebo were tested for romiplostim and TPO antibodies. Prior to romiplostim treatment, 17 subjects (7%) tested romiplostim antibody positive and 12 subjects (5%) tested TPO antibody positive for pre-existing binding antibodies. After romiplostim exposure, 11% of the subjects exhibited binding antibodies against romiplostim and 5% of the subjects with ITP showed binding antibodies against TPO. The antibodies against romiplostim did not cross-react with TPO and vice versa. No cases of anti-TPO neutralizing antibodies were detected in romiplostim-treated subjects. The incidence of anti-romiplostim neutralizing antibodies to romiplostim was 0.4% (one subject); this subject tested negative at the time of follow-up 4 months later. No impact on platelet profiles were apparent in subjects that had antibodies to romiplostim to date. In summary, administration of romiplostim in ITP subjects resulted in the development of a binding antibody response against romiplostim and TPO ligand. One subject developed a neutralizing antibody response to romiplostim that impacted the platelet counts of this subject. No neutralizing antibodies to endogenous TPO were observed.
Figures

Similar articles
-
Assessment of romiplostim immunogenicity in adult patients in clinical trials and in a global postmarketing registry.Br J Haematol. 2020 Sep;190(6):923-932. doi: 10.1111/bjh.16658. Epub 2020 Apr 20. Br J Haematol. 2020. PMID: 32311075 Free PMC article.
-
Romiplostim in adults with newly diagnosed or persistent immune thrombocytopenia.Expert Rev Hematol. 2020 Dec;13(12):1319-1332. doi: 10.1080/17474086.2020.1850253. Epub 2020 Nov 30. Expert Rev Hematol. 2020. PMID: 33249935 Review.
-
Romiplostim in chronic immune thrombocytopenic purpura.Clin Ther. 2009 Sep;31(9):1887-907. doi: 10.1016/j.clinthera.2009.09.013. Clin Ther. 2009. PMID: 19843480 Review.
-
Romiplostim: a review of its use in immune thrombocytopenia.Drugs. 2012 Feb 12;72(3):415-35. doi: 10.2165/11208260-000000000-00000. Drugs. 2012. PMID: 22316355 Review.
-
Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim.Int J Hematol. 2015 Sep;102(3):259-70. doi: 10.1007/s12185-015-1837-6. Epub 2015 Jul 23. Int J Hematol. 2015. PMID: 26201709 Clinical Trial.
Cited by
-
Development of Romiplostim for Treatment of Primary Immune Thrombocytopenia From a Pharmacokinetic and Pharmacodynamic Perspective.Clin Pharmacokinet. 2016 Sep;55(9):1045-58. doi: 10.1007/s40262-016-0382-7. Clin Pharmacokinet. 2016. PMID: 27056734 Review.
-
Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy.AAPS J. 2012 Jun;14(2):296-302. doi: 10.1208/s12248-012-9340-y. Epub 2012 Mar 10. AAPS J. 2012. PMID: 22407289 Free PMC article. Review.
-
Assessment of romiplostim immunogenicity in adult patients in clinical trials and in a global postmarketing registry.Br J Haematol. 2020 Sep;190(6):923-932. doi: 10.1111/bjh.16658. Epub 2020 Apr 20. Br J Haematol. 2020. PMID: 32311075 Free PMC article.
-
A review of immune thrombocytopenic purpura: focus on the novel thrombopoietin agonists.J Blood Med. 2010;1:21-31. doi: 10.2147/JBM.S6803. Epub 2010 Mar 23. J Blood Med. 2010. PMID: 22282680 Free PMC article.
-
Multicenter Single-Blind Randomized Controlled Trial of the Romiplostim Biosimilar.EJHaem. 2025 Jul 24;6(4):e70105. doi: 10.1002/jha2.70105. eCollection 2025 Aug. EJHaem. 2025. PMID: 40708707 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

