In utero exposure of female CD-1 mice to AZT and/or 3TC: II. Persistence of functional alterations in cardiac tissue
- PMID: 20155331
- PMCID: PMC3189686
- DOI: 10.1007/s12012-010-9065-z
In utero exposure of female CD-1 mice to AZT and/or 3TC: II. Persistence of functional alterations in cardiac tissue
Abstract
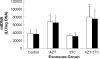
To delineate temporal changes in the integrity and function of mitochondria/cardiomyocytes in hearts from mice exposed in utero to commonly used nucleoside analogs (NRTIs), CD-1 mice were exposed in utero to 80 mg AZT/kg, 40 mg 3TC/kg, 80 mg AZT/kg plus 40 mg 3TC/kg, or vehicle alone during days 12-18 of gestation and hearts from female mouse offspring were examined at 13 and 26 weeks postpartum. Alterations in cardiac mitochondrial DNA (mtDNA) content, oxidative phosphorylation (OXPHOS) enzyme activities, mtDNA mutations, and echocardiography of NRTI-exposed mice were assessed and compared with findings in vehicle-exposed control mice. A hybrid capture-chemiluminescence assay showed significant twofold increases in mtDNA levels in hearts from AZT- and AZT/3TC-exposed mice at 13 and 26 weeks postpartum, consistent with near doubling in mitochondrial numbers over time compared with vehicle-exposed mice. Echocardiographic measurements at 13 and 26 weeks postpartum indicated progressive thinning of the left ventricular posterior wall in NRTI-exposed mice, relative to controls, with differences becoming statistically significant by 26 weeks. Overall, progressive functional changes occurred in mouse mitochondria and cardiac tissue several months after in utero NRTI exposures; AZT and 3TC acted in concert to cause additive cardiotoxic effects of AZT/3TC compared with either drug alone.
Figures
References
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. Journal of Acquired Immune Deficiency Syndromes. 2002;29:484–494. - PubMed
-
- UNAIDS The Joint United Nations Programme on HIV/AIDS. http://www.unaids.org/en/ - PubMed
-
- Watts DH. Treating HIV during pregnancy. An update on safety issues. Drug Safety. 2006;29:467–490. - PubMed
-
- Thorne C, Newell ML. Safety of agents used to prevent mother-to-child transmission of HIV: Is there any cause for concern? Drug Safety. 2007;30:203–213. - PubMed
-
- European Collaborative Study Mother to child transmission of HIV infection in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2005;40:458–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

