A brief history of phytochromes
- PMID: 20155775
- PMCID: PMC2880163
- DOI: 10.1002/cphc.200900894
A brief history of phytochromes
Abstract
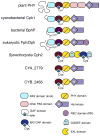
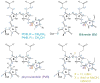
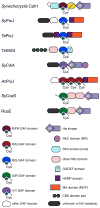
Photosensory proteins enable living things to detect the quantity and quality of the light environment and to transduce that physical signal into biochemical outputs which entrain their metabolism with the ambient light environment. Phytochromes, which photoconvert between red-absorbing P(r) and far-red-absorbing P(fr) states, are the most extensively studied of these interesting proteins. Critical regulators of a number of key adaptive processes in higher plants, including photomorphogenesis and shade avoidance, phytochromes are widespread in photosynthetic and nonphotosynthetic bacteria, and even in fungi. Cyanobacterial genomes also possess a plethora of more distant relatives of phytochromes known as cyanobacteriochromes (CBCRs). Biochemical characterization of representative CBCRs has demonstrated that this class of photosensors exhibits a broad range of wavelength sensitivities, spanning the entire visible spectrum. Distinct protein-bilin interactions are responsible for this astonishing array of wavelength sensitivities. Despite this spectral diversity, all members of the extended family of phytochrome photosensors appear to share a common photochemical mechanism for light sensing: photoisomerization of the 15/16 double bond of the bilin chromophore.
Figures



References
-
- Sage LC. Pigment of the Imagination : A History of Phytochrome Research. Academic Press, Inc; San Diego: 1992.
-
- Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R. Curr Biol. 2005;15:1833–1838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

