Region-specific protein abundance changes in the brain of MPTP-induced Parkinson's disease mouse model
- PMID: 20155936
- PMCID: PMC2859700
- DOI: 10.1021/pr901024z
Region-specific protein abundance changes in the brain of MPTP-induced Parkinson's disease mouse model
Abstract
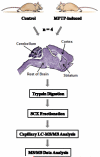
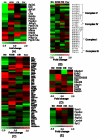
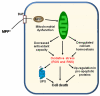
Parkinson's disease (PD) is characterized by dopaminergic neurodegeneration in the nigrostriatal region of the brain; however, the neurodegeneration extends well beyond dopaminergic neurons. To gain a better understanding of the molecular changes relevant to PD, we applied two-dimensional LC-MS/MS to comparatively analyze the proteome changes in four brain regions (striatum, cerebellum, cortex, and the rest of brain) using a MPTP-induced PD mouse model with the objective to identify potential nigrostriatal-specific and other region-specific protein abundance changes. The combined analyses resulted in the identification of 4,895 nonredundant proteins with at least two unique peptides per protein. The relative abundance changes in each analyzed brain region were estimated based on the spectral count information. A total of 518 proteins were observed with substantial MPTP-induced abundance changes across different brain regions. A total of 270 of these proteins were observed with specific changes occurring either only in the striatum and/or in the rest of the brain region that contains substantia nigra, suggesting that these proteins are associated with the underlying nigrostriatal pathways. Many of the proteins that exhibit changes were associated with dopamine signaling, mitochondrial dysfunction, the ubiquitin system, calcium signaling, the oxidative stress response, and apoptosis. A set of proteins with either consistent change across all brain regions or with changes specific to the cortex and cerebellum regions were also detected. Ubiquitin specific protease (USP9X), a deubiquination enzyme involved in the protection of proteins from degradation and promotion of the TGF-beta pathway, exhibited altered abundance in all brain regions. Western blot validation showed similar spatial changes, suggesting that USP9X is potentially associated with neurodegeneration. Together, this study for the first time presents an overall picture of proteome changes underlying both nigrostriatal pathways and other brain regions potentially involved in MPTP-induced neurodegeneration. The observed molecular changes provide a valuable reference resource for future hypothesis-driven functional studies of PD.
Figures





Similar articles
-
The role of the MYD88-dependent pathway in MPTP-induced brain dopaminergic degeneration.J Neuroinflammation. 2011 Oct 11;8:137. doi: 10.1186/1742-2094-8-137. J Neuroinflammation. 2011. PMID: 21989292 Free PMC article.
-
Cholinergic and Dopaminergic Alterations in Nigrostriatal Neurons Are Involved in Environmental Enrichment Motor Protection in a Mouse Model of Parkinson's Disease.J Mol Neurosci. 2016 Dec;60(4):453-464. doi: 10.1007/s12031-016-0831-7. Epub 2016 Sep 22. J Mol Neurosci. 2016. PMID: 27660217
-
Brain Selective Estrogen Treatment Protects Dopaminergic Neurons and Preserves Behavioral Function in MPTP-induced Mouse Model of Parkinson's Disease.J Neuroimmune Pharmacol. 2021 Sep;16(3):667-678. doi: 10.1007/s11481-020-09972-1. Epub 2020 Nov 22. J Neuroimmune Pharmacol. 2021. PMID: 33221984 Free PMC article.
-
Mechanisms of MPTP toxicity and their implications for therapy of Parkinson's disease.Med Sci Monit. 2005 Jan;11(1):RA17-23. Med Sci Monit. 2005. PMID: 15614202 Review.
-
Dual spatial maps of transcript and protein abundance in the mouse brain.Expert Rev Proteomics. 2009 Jun;6(3):243-9. doi: 10.1586/epr.09.46. Expert Rev Proteomics. 2009. PMID: 19489697 Free PMC article. Review.
Cited by
-
Systems biology analysis of the proteomic alterations induced by MPP(+), a Parkinson's disease-related mitochondrial toxin.Front Cell Neurosci. 2015 Feb 2;9:14. doi: 10.3389/fncel.2015.00014. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25698928 Free PMC article.
-
Balancing act: deubiquitinating enzymes in the nervous system.Trends Neurosci. 2011 Jul;34(7):370-82. doi: 10.1016/j.tins.2011.05.004. Epub 2011 Jun 24. Trends Neurosci. 2011. PMID: 21704388 Free PMC article. Review.
-
Neurotrophic Effect of Asiatic acid, a Triterpene of Centella asiatica Against Chronic 1-Methyl 4-Phenyl 1, 2, 3, 6-Tetrahydropyridine Hydrochloride/Probenecid Mouse Model of Parkinson's disease: The Role of MAPK, PI3K-Akt-GSK3β and mTOR Signalling Pathways.Neurochem Res. 2017 May;42(5):1354-1365. doi: 10.1007/s11064-017-2183-2. Epub 2017 Feb 8. Neurochem Res. 2017. PMID: 28181071
-
Monoamine Oxidase Inhibitors in Toxic Models of Parkinsonism.Int J Mol Sci. 2025 Jan 31;26(3):1248. doi: 10.3390/ijms26031248. Int J Mol Sci. 2025. PMID: 39941014 Free PMC article. Review.
-
Altered expression of Sialyl Lewis X in experimental models of Parkinson's disease.J Mol Med (Berl). 2024 Mar;102(3):365-377. doi: 10.1007/s00109-023-02415-3. Epub 2024 Jan 10. J Mol Med (Berl). 2024. PMID: 38197965 Free PMC article.
References
-
- Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol. 1998;44:S72–84. - PubMed
-
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. - PubMed
-
- Forno LS, DeLanney LE, Irwin I, Langston JW. Electron microscopy of Lewy bodies in the amygdala-parahippocampal region. Comparison with inclusion bodies in the MPTP-treated squirrel monkey. Adv Neurol. 1996;69:217–228. - PubMed
-
- Parkinson J. an essay on the shaking palsy. J. Neuropsychiatry Clin. Neurosci. 1817:223–236. - PubMed
-
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

