Genetic characterization of HIV type 1 Nef-induced vesicle secretion
- PMID: 20156100
- PMCID: PMC2835390
- DOI: 10.1089/aid.2009.0068
Genetic characterization of HIV type 1 Nef-induced vesicle secretion
Abstract
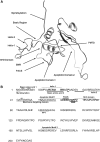
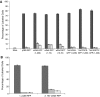
The HIV-1 Nef protein is known to be secreted, and our group has shown that Nef is secreted from nef-transfected and HIV-1-infected cells in small exosome-like vesicles (d. 40-100 nm). The role of secreted Nef remains to be fully characterized. Thus, it is important to characterize the nature of and the mechanisms regulating Nef secretion. We hypothesized that specific structural domains on the Nef protein interact with components of the endosomal trafficking machinery, sorting Nef into multivesicular bodies (MVB) and packaging it in exosome-like vesicles. To identify those domains, a series of mutants spanning the entire nef sequence were made and cloned into the expression vector pQB1, which expresses the mutants as Nef-GFP fusion proteins. These constructs were used in transient transfection assays to identify sequences necessary for secretion of the Nef-GFP fusion protein. N-terminal domains were identified as critical for Nef-induced vesicle secretion: (1) a basic cluster of four arginine residues (aa 17, 19, 21, 22), (2) the phosphofurin acidic cluster sequence (PACS; Glu62-65), and (3) a previously uncharacterized domain spanning amino acid residues 66-70 (VGFPV), which we named the secretion modification region (SMR). Additional amino acids P25, 29GVG31, and T44 were identified in HIV-1 Nef as regulating its secretion. These residues have not been associated with other reported Nef functions. The myristoylation domain, ubiquitination lysine residues, and the C-terminal portion of Nef (aa 71-206) had no effect on secretion. A minimal HIV-1 Nef sequence, comprising the identified motifs, was sufficient for Nef-induced vesicle secretion.
Figures







References
-
- Ho DD. Neumann AU. Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. - PubMed
-
- Wei X. Ghosh SK. Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373(6510):117–122. - PubMed
-
- Perelson AS. Neumann AU. Markowitz M. Leonard JM. Ho DD. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–1586. - PubMed
-
- Bagasra O. Hauptman SP. Lischner HW. Sachs M. Pomerantz RJ. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992;326(21):1385–1391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

