Functional cooperation between FACT and MCM is coordinated with cell cycle and differential complex formation
- PMID: 20156367
- PMCID: PMC2848000
- DOI: 10.1186/1423-0127-17-11
Functional cooperation between FACT and MCM is coordinated with cell cycle and differential complex formation
Abstract
Background: Functional cooperation between FACT and the MCM helicase complex constitutes an integral step during DNA replication initiation. However, mode of regulation that underlies the proper functional interaction of FACT and MCM is poorly understood.
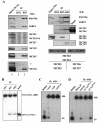
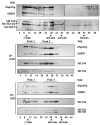
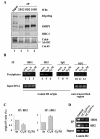
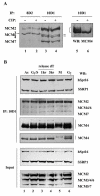
Methods & results: Here we present evidence indicating that such interaction is coordinated with cell cycle progression and differential complex formation. We first demonstrate the existence of two distinct FACT-MCM subassemblies, FACT-MCM2/4/6/7 and FACT-MCM2/3/4/5. Both complexes possess DNA unwinding activity and are subject to cell cycle-dependent enzymatic regulation. Interestingly, analysis of functional attributes further suggests that they act at distinct, and possibly sequential, steps during origin establishment and replication initiation. Moreover, we show that the phosphorylation profile of the FACT-associated MCM4 undergoes a cell cycle-dependent change, which is directly correlated with the catalytic activity of the FACT-MCM helicase complexes. Finally, at the quaternary structure level, physical interaction between FACT and MCM complexes is generally dependent on persistent cell cycle and further stabilized upon S phase entry. Cessation of mitotic cycle destabilizes the complex formation and likely leads to compromised coordination and activities.
Conclusions: Together, our results correlate FACT-MCM functionally and temporally with S phase and DNA replication. They further demonstrate that enzymatic activities intrinsically important for DNA replication are tightly controlled at various levels, thereby ensuring proper progression of, as well as exit from, the cell cycle and ultimately euploid gene balance.
Figures

Similar articles
-
Functional cooperation between FACT and MCM helicase facilitates initiation of chromatin DNA replication.EMBO J. 2006 Sep 6;25(17):3975-85. doi: 10.1038/sj.emboj.7601271. Epub 2006 Aug 10. EMBO J. 2006. PMID: 16902406 Free PMC article.
-
The histone chaperone facilitates chromatin transcription (FACT) protein maintains normal replication fork rates.J Biol Chem. 2011 Sep 2;286(35):30504-30512. doi: 10.1074/jbc.M111.264721. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21757688 Free PMC article.
-
Cdt1 forms a complex with the minichromosome maintenance protein (MCM) and activates its helicase activity.J Biol Chem. 2008 Sep 5;283(36):24469-77. doi: 10.1074/jbc.M803212200. Epub 2008 Jul 7. J Biol Chem. 2008. PMID: 18606811 Free PMC article.
-
The Mcm complex: unwinding the mechanism of a replicative helicase.Microbiol Mol Biol Rev. 2009 Dec;73(4):652-83. doi: 10.1128/MMBR.00019-09. Microbiol Mol Biol Rev. 2009. PMID: 19946136 Free PMC article. Review.
-
Control of DNA replication: regulation and activation of eukaryotic replicative helicase, MCM.IUBMB Life. 2005 Apr-May;57(4-5):323-35. doi: 10.1080/15216540500092419. IUBMB Life. 2005. PMID: 16036617 Review.
Cited by
-
Epigenetic landscape for initiation of DNA replication.Chromosoma. 2014 Jun;123(3):183-99. doi: 10.1007/s00412-013-0448-3. Epub 2013 Dec 17. Chromosoma. 2014. PMID: 24337246 Review.
-
FACT complex gene duplicates exhibit redundant and non-redundant functions in C. elegans.Dev Biol. 2018 Dec 15;444(2):71-82. doi: 10.1016/j.ydbio.2018.10.002. Epub 2018 Oct 15. Dev Biol. 2018. PMID: 30336114 Free PMC article.
-
SSRP1 silencing inhibits the proliferation and malignancy of human glioma cells via the MAPK signaling pathway.Oncol Rep. 2017 Nov;38(5):2667-2676. doi: 10.3892/or.2017.5982. Epub 2017 Sep 21. Oncol Rep. 2017. PMID: 29048646 Free PMC article.
-
Modulation of chromatin structure by the FACT histone chaperone complex regulates HIV-1 integration.Retrovirology. 2017 Jul 28;14(1):39. doi: 10.1186/s12977-017-0363-4. Retrovirology. 2017. PMID: 28754126 Free PMC article.
-
Histone chaperone FACT is essential to overcome replication stress in mammalian cells.Oncogene. 2020 Jul;39(28):5124-5137. doi: 10.1038/s41388-020-1346-9. Epub 2020 Jun 12. Oncogene. 2020. PMID: 32533099 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

