Pro-2-PAM therapy for central and peripheral cholinesterases
- PMID: 20156430
- PMCID: PMC2889221
- DOI: 10.1016/j.cbi.2010.02.015
Pro-2-PAM therapy for central and peripheral cholinesterases
Abstract
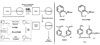
Novel therapeutics to overcome the toxic effects of organophosphorus (OP) chemical agents are needed due to the documented use of OPs in warfare (e.g. 1980-1988 Iran/Iraq war) and terrorism (e.g. 1995 Tokyo subway attacks). Standard OP exposure therapy in the United States consists of atropine sulfate (to block muscarinic receptors), the acetylcholinesterase (AChE) reactivator (oxime) pralidoxime chloride (2-PAM), and a benzodiazepine anticonvulsant to ameliorate seizures. A major disadvantage is that quaternary nitrogen charged oximes, including 2-PAM, do not cross the blood brain barrier (BBB) to treat brain AChE. Therefore, we have synthesized and evaluated pro-2-PAM (a lipid permeable 2-PAM derivative) that can enter the brain and reactivate CNS AChE, preventing seizures in guinea pigs after exposure to OPs. The protective effects of the pro-2-PAM after OP exposure were shown using (a) surgically implanted radiotelemetry probes for electroencephalogram (EEG), (b) neurohistopathology of brain, (c) cholinesterase activities in the PNS and CNS, and (d) survivability. The PNS oxime 2-PAM was ineffective at reducing seizures/status epilepticus (SE) in diisopropylfluorophosphate (DFP)-exposed animals. In contrast, pro-2-PAM significantly suppressed and then eliminated seizure activity. In OP-exposed guinea pigs, there was a significant reduction in neurological damage with pro-2-PAM but not 2-PAM. Distinct regional areas of the brains showed significantly higher AChE activity 1.5h after OP exposure in pro-2-PAM treated animals compared to the 2-PAM treated ones. However, blood and diaphragm showed similar AChE activities in animals treated with either oxime, as both 2-PAM and pro-2-PAM are PNS active oximes. In conclusion, pro-2-PAM can cross the BBB, is rapidly metabolized inside the brain to 2-PAM, and protects against OP-induced SE through restoration of brain AChE activity. Pro-2-PAM represents the first non-invasive means of administering a CNS therapeutic for the deleterious effects of OP poisoning by reactivating CNS AChE.
Published by Elsevier Ireland Ltd.
Figures





References
-
- Taylor P. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 8th edition. Gilman AG, Rall TW, Neis AS, Taylor P, editors. 1990. pp. 131–149.
-
- Gordon RK, Clarkson ED Rapid Decontamination of Chemical Warfare Agents. In: Handbook of Toxicology of Chemical Warfare Agents. Gupta RC, editor. Vol. 71. 2009. pp. 1069–1081.
-
- Grob D, Harvey AM. The effects and treatment of nerve gas poisoning. Am J Med. 1953;14:52–63. - PubMed
-
- Carpentier P, Foquin A, Rondouin G, Lerner-Natoli M, De Groot D, Lallement G. Effects of atropine sulphate on seizure activity and brain damage produced by soman in guinea-pigs: EcoG correlates of neuropathology. Neurotoxicol. 2000;21(4):521–540. - PubMed
-
- Hatta K, Miura Y, Asukai N, Hamabe Y. Amnesia from sarin poisoning. Lancet. 1996;347:1343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

