Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions
- PMID: 20156436
- PMCID: PMC2878840
- DOI: 10.1016/j.yexcr.2010.02.008
Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions
Abstract
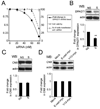
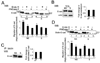
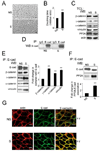
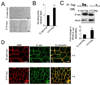
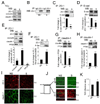
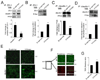
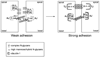
Epithelial cell-cell adhesion is controlled by multiprotein complexes that include E-cadherin-mediated adherens junctions (AJs) and ZO-1-containing tight junctions (TJs). Previously, we reported that reduction of E-cadherin N-glycosylation in normal and cancer cells promoted stabilization of AJs through changes in the composition and cytoskeletal association of E-cadherin scaffolds. Here, we show that enhanced interaction of hypoglycosylated E-cadherin-containing AJs with protein phosphatase 2A (PP2A) represents a mechanism for promoting TJ assembly. In MDCK cells, attenuation of cellular N-glycosylation with siRNA to DPAGT1, the first gene in the N-glycosylation pathway, reduced N-glycosylation of surface E-cadherin and resulted in increased recruitment of stabilizing proteins gamma-catenin, alpha-catenin, vinculin and PP2A to AJs. Greater association of PP2A with AJs correlated with diminished binding of PP2A to ZO-1 and claudin-1 and with increased pools of serine-phosphorylated ZO-1 and claudin-1. More ZO-1 was found in complexes with occludin and claudin-1, and this corresponded to enhanced transepithelial resistance (TER), indicating physiological assembly of TJs. Similar maturation of AJs and TJs was detected after transfection of MDCK cells with the hypoglycosylated E-cadherin variant, V13. Our data indicate that E-cadherin N-glycans coordinate the maturity of AJs with the assembly of TJs by affecting the association of PP2A with these junctional complexes.
Figures
Similar articles
-
N-glycosylation status of E-cadherin controls cytoskeletal dynamics through the organization of distinct β-catenin- and γ-catenin-containing AJs.Cell Health Cytoskelet. 2009 Sep 16;2009(1):67-80. doi: 10.2147/chc.s5965. Cell Health Cytoskelet. 2009. PMID: 20502620 Free PMC article.
-
Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells.J Biol Chem. 2010 Feb 12;285(7):5003-12. doi: 10.1074/jbc.M109.043760. Epub 2009 Dec 12. J Biol Chem. 2010. PMID: 20008323 Free PMC article.
-
Coordinate regulation of N-glycosylation gene DPAGT1, canonical Wnt signaling and E-cadherin adhesion.J Cell Sci. 2013 Jan 15;126(Pt 2):484-96. doi: 10.1242/jcs.113035. Epub 2012 Nov 23. J Cell Sci. 2013. PMID: 23178939 Free PMC article.
-
MicroRNA Regulation of Endothelial Junction Proteins and Clinical Consequence.Mediators Inflamm. 2016;2016:5078627. doi: 10.1155/2016/5078627. Epub 2016 Nov 24. Mediators Inflamm. 2016. PMID: 27999452 Free PMC article. Review.
-
Adherens and tight junctions: structure, function and connections to the actin cytoskeleton.Biochim Biophys Acta. 2008 Mar;1778(3):660-9. doi: 10.1016/j.bbamem.2007.07.012. Epub 2007 Jul 27. Biochim Biophys Acta. 2008. PMID: 17854762 Free PMC article. Review.
Cited by
-
N‑glycosylation and receptor tyrosine kinase signaling affect claudin‑3 levels in colorectal cancer cells.Oncol Rep. 2020 Oct;44(4):1649-1661. doi: 10.3892/or.2020.7727. Epub 2020 Aug 11. Oncol Rep. 2020. PMID: 32945502 Free PMC article.
-
Modulation of E-cadherin function and dysfunction by N-glycosylation.Cell Mol Life Sci. 2011 Mar;68(6):1011-20. doi: 10.1007/s00018-010-0595-0. Epub 2010 Nov 23. Cell Mol Life Sci. 2011. PMID: 21104290 Free PMC article. Review.
-
The Misregulation of Cell Adhesion Components during Tumorigenesis: Overview and Commentary.J Oncol. 2010;2010:174715. doi: 10.1155/2010/174715. Epub 2010 Sep 30. J Oncol. 2010. PMID: 20953359 Free PMC article.
-
Protein Ser/Thr phosphatase-6 is required for maintenance of E-cadherin at adherens junctions.BMC Cell Biol. 2013 Sep 25;14:42. doi: 10.1186/1471-2121-14-42. BMC Cell Biol. 2013. PMID: 24063632 Free PMC article.
-
Protein Phosphatase 2A: More Than a Passenger in the Regulation of Epithelial Cell-Cell Junctions.Front Cell Dev Biol. 2019 Mar 6;7:30. doi: 10.3389/fcell.2019.00030. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 30895176 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

