Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions
- PMID: 20156436
- PMCID: PMC2878840
- DOI: 10.1016/j.yexcr.2010.02.008
Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions
Abstract
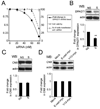
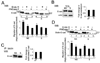
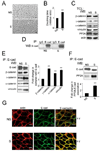
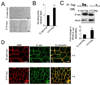
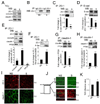
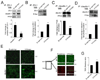
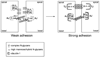
Epithelial cell-cell adhesion is controlled by multiprotein complexes that include E-cadherin-mediated adherens junctions (AJs) and ZO-1-containing tight junctions (TJs). Previously, we reported that reduction of E-cadherin N-glycosylation in normal and cancer cells promoted stabilization of AJs through changes in the composition and cytoskeletal association of E-cadherin scaffolds. Here, we show that enhanced interaction of hypoglycosylated E-cadherin-containing AJs with protein phosphatase 2A (PP2A) represents a mechanism for promoting TJ assembly. In MDCK cells, attenuation of cellular N-glycosylation with siRNA to DPAGT1, the first gene in the N-glycosylation pathway, reduced N-glycosylation of surface E-cadherin and resulted in increased recruitment of stabilizing proteins gamma-catenin, alpha-catenin, vinculin and PP2A to AJs. Greater association of PP2A with AJs correlated with diminished binding of PP2A to ZO-1 and claudin-1 and with increased pools of serine-phosphorylated ZO-1 and claudin-1. More ZO-1 was found in complexes with occludin and claudin-1, and this corresponded to enhanced transepithelial resistance (TER), indicating physiological assembly of TJs. Similar maturation of AJs and TJs was detected after transfection of MDCK cells with the hypoglycosylated E-cadherin variant, V13. Our data indicate that E-cadherin N-glycans coordinate the maturity of AJs with the assembly of TJs by affecting the association of PP2A with these junctional complexes.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

