Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity
- PMID: 20156488
- PMCID: PMC2918371
- DOI: 10.1016/j.bbr.2010.02.016
Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity
Abstract
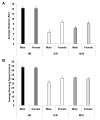
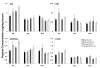
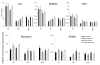
Dysregulation of the dopamine system is linked to various aberrant behaviors, including addiction, compulsive exercise, and hyperphagia leading to obesity. The goal of the present experiments was to determine how dopamine contributes to the expression of opposing phenotypes, excessive exercise and obesity. We hypothesized that similar alterations in dopamine and dopamine-related gene expression may underly obesity and excessive exercise, as competing traits for central reward pathways. Moreover, we hypothesized that selective breeding for high levels of exercise or obesity may have influenced genetic variation controlling these pathways, manifesting as opposing complex traits. Dopamine, dopamine-related peptide concentrations, and gene expression were evaluated in dorsal striatum (DS) and nucleus accumbens (NA) of mice from lines selectively bred for high rates of wheel running (HR) or obesity (M16), and the non-selected ICR strain from which these lines were derived. HPLC analysis showed significantly greater neurotransmitter concentrations in DS and NA of HR mice compared to M16 and ICR. Microarray analysis showed significant gene expression differences between HR and M16 compared to ICR in both brain areas, with changes revealed throughout the dopamine pathway including D1 and D2 receptors, associated G-proteins (e.g., Golf), and adenylate cyclase (e.g., Adcy5). The results suggest that similar modifications within the dopamine system may contribute to the expression of opposite phenotypes in mice, demonstrating that alterations within central reward pathways can contribute to both obesity and excessive exercise.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Genotypic differences in brain dopamine receptor function in the DBA/2J and C57BL/6J inbred mouse strains.Eur J Pharmacol. 1994 Nov 15;269(3):349-64. doi: 10.1016/0922-4106(94)90043-4. Eur J Pharmacol. 1994. PMID: 7895774
-
[Age-related changes in behavior, in monoamines and their metabolites content, and in density of D1 and D2 dopamine receptors in the brain structures of WAG/Rij rats with depression-like pathology].Zh Vyssh Nerv Deiat Im I P Pavlova. 2014 Nov-Dec;64(6):668-85. Zh Vyssh Nerv Deiat Im I P Pavlova. 2014. PMID: 25975143 Russian.
-
Exercise-Induced Adaptations to the Mouse Striatal Adenosine System.Neural Plast. 2020 Jan 28;2020:5859098. doi: 10.1155/2020/5859098. eCollection 2020. Neural Plast. 2020. PMID: 32399024 Free PMC article.
-
The role of dopamine in overcoming aversion with exercise.Brain Res. 2019 Jun 15;1713:102-108. doi: 10.1016/j.brainres.2018.08.030. Epub 2018 Aug 29. Brain Res. 2019. PMID: 30171838 Review.
-
Molecular characteristics of mammalian dopamine receptors.Pharmacol Toxicol. 1997 Sep;81(3):105-13. doi: 10.1111/j.1600-0773.1997.tb00039.x. Pharmacol Toxicol. 1997. PMID: 9335067 Review.
Cited by
-
The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives.J Exp Biol. 2011 Jan 15;214(Pt 2):206-29. doi: 10.1242/jeb.048397. J Exp Biol. 2011. PMID: 21177942 Free PMC article. Review.
-
The effects of short-term high-fat feeding on exercise capacity: multi-tissue transcriptome changes by RNA sequencing analysis.Lipids Health Dis. 2017 Feb 2;16(1):28. doi: 10.1186/s12944-017-0424-7. Lipids Health Dis. 2017. PMID: 28153015 Free PMC article.
-
Obesity-Related Genetic Variants and their Associations with Physical Activity.Sports Med Open. 2015;1(1):34. doi: 10.1186/s40798-015-0036-6. Epub 2015 Oct 15. Sports Med Open. 2015. PMID: 26495240 Free PMC article.
-
Neurobiological studies of fatigue.Prog Neurobiol. 2012 Nov;99(2):93-105. doi: 10.1016/j.pneurobio.2012.07.004. Epub 2012 Jul 24. Prog Neurobiol. 2012. PMID: 22841649 Free PMC article. Review.
-
A Comprehensive Inter-Tissue Crosstalk Analysis Underlying Progression and Control of Obesity and Diabetes.Sci Rep. 2015 Jul 23;5:12340. doi: 10.1038/srep12340. Sci Rep. 2015. PMID: 26202695 Free PMC article.
References
-
- Allan MF, Eisen EJ, Pomp D. The M16 mouse: an outbred animal model of early onset polygenic obesity and diabesity. Obes Res. 2004;12:1397–407. - PubMed
-
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;678(Suppl):7–12. - PubMed
-
- Belke TW. Responding for sucrose and wheel-running reinforcement: effect of pre-running. Behav Processes. 2006a;71:1–7. - PubMed
-
- Belke TW. Concurrent schedules of wheel-running reinforcement: choice between different durations of opportunity to run in rats. Learn Behav. 2006b;34:61–70. - PubMed
-
- Belke TW, Christie-Fougere MM. Investigations of timing during the schedule and reinforcement intervals with wheel-running reinforcement. Behav Processes. 2006;73:240–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

